��Ŀ����
ͼ2Ϊ�����и���Ӫ�����ʵĺ������������˵���������������ŷdz���Ҫ�����ã�
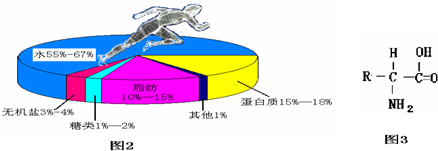
��1�����������һ�����ϵ�������Դ��______��
��2��ά��������������һ��Ӫ���أ�ά���ص�����ܶ࣬����ά����A�ֽ��ӻƴ�������ȱ��ά����A�Ỽ______������ţ���ҹä֢���ڻ�Ѫ���������Dz�
��3�������������������ʻ��������������ɶ��ְ����ṹ�ɵģ����ְ�����Ľṹ����ͼ3��ʾ����������һ�����е�Ԫ����______��
��4������Ԫ���������еĺ�������̫�ٶ���Ӱ�����彡����������Ԫ�����Ԫ�ز�������Ľ�����������
Ca Ӱ������
Fe �鴤�����Dz�
Zn ƶѪ�����Զ�����

��1�����������һ�����ϵ�������Դ��______��
��2��ά��������������һ��Ӫ���أ�ά���ص�����ܶ࣬����ά����A�ֽ��ӻƴ�������ȱ��ά����A�Ỽ______������ţ���ҹä֢���ڻ�Ѫ���������Dz�
��3�������������������ʻ��������������ɶ��ְ����ṹ�ɵģ����ְ�����Ľṹ����ͼ3��ʾ����������һ�����е�Ԫ����______��
��4������Ԫ���������еĺ�������̫�ٶ���Ӱ�����彡����������Ԫ�����Ԫ�ز�������Ľ�����������
Ca Ӱ������
Fe �鴤�����Dz�
Zn ƶѪ�����Զ�����
��1���������������������Ҫ�������ʣ�һ�����ϵ�������Դ�� ���࣮
��2������ȱ��ά����A�Ỽҹä֢
��3���ɰ�����Ľṹͼ��֪����������һ�����е�Ԫ���� C��H��O��N��
��4������ȱ�ƻ�鴤�����Dz���ȱ����ƶѪ�����Զ�����ȱп��Ӱ������
�ʴ�Ϊ����1�����ࣻ
��2���٣�
��3��C��H��O��N��
��4��

��2������ȱ��ά����A�Ỽҹä֢
��3���ɰ�����Ľṹͼ��֪����������һ�����е�Ԫ���� C��H��O��N��
��4������ȱ�ƻ�鴤�����Dz���ȱ����ƶѪ�����Զ�����ȱп��Ӱ������
�ʴ�Ϊ����1�����ࣻ
��2���٣�
��3��C��H��O��N��
��4��


��ϰ��ϵ�д�
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ

 ��2012?��Զ�ж�ģ��������������벻����ѧ��
��2012?��Զ�ж�ģ��������������벻����ѧ��





