��Ŀ����
ijͬѧ��ѧϰ��Һ�������ʱ��������ҺNaCl������Na2CO3��Һ�ʼ��ԣ���ͬѧ��Ϊ�п����е�����Һ������ԣ����������Ϸ��֣�NH4��2SO4��FeCl3��Һ�����ԣ���ͬѧ����Ȥ��д�����µ�ѧϰС�ᣬ������һ����
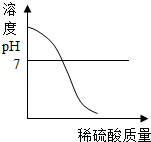
��1������ҺpH�Ŀ���Ϊ������ļ�������ո��У����� ������ ����pH=7
��2��Ҫ�ⶨ����Һ����������� ������Ҫ֪������Һ�����ȿ����� �������������� ����
��3����ׯ��һ֦����ȫ���ʵ��ң�����NH4��2SO4��һ�ֵ����ܴٽ�ũ����ľ���Ҷ����ïʢ����ʹ��ɫʯ����Һ���� ��ɫ��
��4���̬���ʵļ�ⷽ���ǣ��� ����
��5����ͬѧ����FeCl3��Һ�����к��ɫ�������ɣ���д����ѧ����ʽ���� ��������ʾ��Cu��OH��2�������Լ���pH��5�����в��ܽ⣩
��6��ΪʲôNa2CO3��Һ�ʼ��ԣ���NH4��2SO4��FeCl3��Һ�����ԣ������鲻�Ǻ����ף��Ǹ���Ҫѧ�ģ����ϸ��к���һ��Ҫ�������
��1������ҺpH�Ŀ���Ϊ������ļ�������ո��У����� ������ ����pH=7
��2��Ҫ�ⶨ����Һ����������� ������Ҫ֪������Һ�����ȿ����� �������������� ����
��3����ׯ��һ֦����ȫ���ʵ��ң�����NH4��2SO4��һ�ֵ����ܴٽ�ũ����ľ���Ҷ����ïʢ����ʹ��ɫʯ����Һ���� ��ɫ��
��4���̬���ʵļ�ⷽ���ǣ��� ����
��5����ͬѧ����FeCl3��Һ�����к��ɫ�������ɣ���д����ѧ����ʽ���� ��������ʾ��Cu��OH��2�������Լ���pH��5�����в��ܽ⣩
��6��ΪʲôNa2CO3��Һ�ʼ��ԣ���NH4��2SO4��FeCl3��Һ�����ԣ������鲻�Ǻ����ף��Ǹ���Ҫѧ�ģ����ϸ��к���һ��Ҫ�������
��1��pH��7��pH��7
��2�����ָʾ����pH��ֽ���ò�����պȡ����ҺͿ��pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����ռ���
��3���� ��4������ʯ�һ����ĥ���۲��Ƿ��д̼��Ե���ζ����
��5��FeCl3+3H20 Fe��OH��3��+3HCl
Fe��OH��3��+3HCl
��2�����ָʾ����pH��ֽ���ò�����պȡ����ҺͿ��pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����ռ���
��3���� ��4������ʯ�һ����ĥ���۲��Ƿ��д̼��Ե���ζ����
��5��FeCl3+3H20
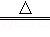 Fe��OH��3��+3HCl
Fe��OH��3��+3HCl�����������1��pH��7����Һ�ʼ��ԣ�pH��7����Һ�����ԣ�pH=7����Һ�����ԣ�����Һ���ܳʼ��ԡ����Ի����ԣ����pH��7��pH��7��
��2�����ָʾ�����Բⶨ��Һ������ԣ��ⶨ��Һ������ʹ�õ���pH��ֽ���ⶨʱҪ�ò�����պȡ����ҺͿ��pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����ռ��ɣ�������ָʾ����pH��ֽ���ò�����պȡ����ҺͿ��pH��ֽ�ϣ�����ʾ����ɫ�����ɫ�����ռ��ɣ�
��3����NH4��2SO4��Һ�����ԣ�������Һ��ʹʯ����Һ��죬����죻
��4���̬������������ʻ�ϻ�����д̼�����ζ�İ������ʿ���ʹ������ʯ�һ����ĥ�ķ������м����̬���ʣ��������ʯ�һ����ĥ���۲��Ƿ��д̼��Ե���ζ������
��5�������Ȼ�����Һ���ɺ��ɫ�����������Ȼ�����ˮ��Ӧ���������������������Ե�ʣ���������ṩ����Ϣ����֪������pH��5��������Һ�������Լ�ܽ⣬���FeCl3+3H20
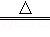 Fe��OH��3��+3HCl��
Fe��OH��3��+3HCl�����������⿼���˳����ε������Լ��й���Һ����ԵIJⶨ����ɴ��⣬�����������е�֪ʶ����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ