��Ŀ����
����Ŀ���ҹ����������������У��Կ����ж��������Ũ�ȱ����±���ʾ������������
�� | һ���� | ������ | ������ |
����������SO2������(mg/m3) | 0~0.15 | 0.15~0.50 | 0.50~0.70 |
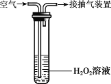
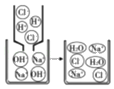
Ϊ�ⶨij���������ж�������ĺ���������С��ͬѧ����ͼ��ʾװ�ý���ʵ�飺ȡ1m3�Ŀ�����ͨ��ʢ������H2O2��Һ�ij���װ�ã���������ʹ���������ܹ���ַ�Ӧ�������ᡣ�����պ��ˮ��Һ�м���������BaCl2��Һ�����ɰ�ɫ����BaSO4����Է�������Ϊ233�������ⶨ�����ɰ�ɫ����������Ϊ1.165mg��
��ͨ�����㣬�жϴ˿����ж��������Ũ�ȼ���__________��
��֪��Ӧ����SO2 + H2O2 = H2SO4��
��H2SO4 +BaCl2 = BaSO4��+ 2HCl��
���𰸡�����
��������
�⣺�����Ȼ�����Ӧ�����������Ϊx��
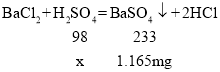
![]()
��ã�x=0.49mg
��1m3�����к�SO2������Ϊy
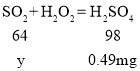
![]()
��ã�y=0.32mg
���Կ����ж��������Ũ�ȼ����Ƕ���
�𣺿����ж��������Ũ�ȼ����Ƕ�����

��ϰ��ϵ�д�
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
�����Ŀ