题目内容
某兴趣小组同学为了检测某石灰石样品中碳酸钙的质量分数,采取的方法是:取样品6g,磨成粉后放入烧杯中,加入足量的稀盐酸,搅拌使其充分反应.反应结束后,测得烧杯内剩余物质的质量减少了2.2g.(已知石灰石样品中的杂质不与盐酸反应,反应的化学方程式为:CaCO3+2HCl═CaCl2+H2O+CO2↑)试求:
(1)根据质量守恒定律,生成二氧化碳的质量是______g
(2)样品中碳酸钙的质量分数.
解:(1)根据质量守恒定律可知,生成二氧化碳的质量就是烧杯内物质减少的质量,故答案为:2.2;
(2)解:设6g石灰石样品中碳酸钙的质量为x
CaCO3+2HCl═CaCl2+H2O+CO2↑
100 44
x 2.2 g

x=5g
石灰石中碳酸钙的质量分数为: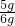 ×100%=83.3%
×100%=83.3%
答:样品中碳酸钙的质量分数为83.3%.
分析:首先根据质量守恒定律求出生成的二氧化碳的质量即烧杯内物质减少的质量,然后把二氧化碳的质量代入化学方程式计算就可求出参与反应的碳酸钙的质量,就可以进一步求出石灰石样品中碳酸钙的质量分数.
点评:本题主要考查了质量守恒定律的应用和根据化学方程式进行有关的计算,要注意解题的步骤和计算结果的准确性,此题难度不大.
(2)解:设6g石灰石样品中碳酸钙的质量为x
CaCO3+2HCl═CaCl2+H2O+CO2↑
100 44
x 2.2 g

x=5g
石灰石中碳酸钙的质量分数为:
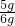 ×100%=83.3%
×100%=83.3%答:样品中碳酸钙的质量分数为83.3%.
分析:首先根据质量守恒定律求出生成的二氧化碳的质量即烧杯内物质减少的质量,然后把二氧化碳的质量代入化学方程式计算就可求出参与反应的碳酸钙的质量,就可以进一步求出石灰石样品中碳酸钙的质量分数.
点评:本题主要考查了质量守恒定律的应用和根据化学方程式进行有关的计算,要注意解题的步骤和计算结果的准确性,此题难度不大.

练习册系列答案
相关题目
下图是实验室制取和收集气体的装置图.
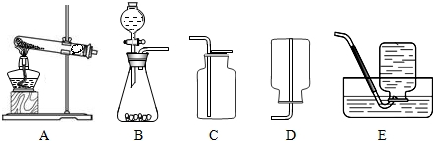
(1)图中有标号的仪器名称:① ;② .
(2)要在实验室中制取并收集一瓶比较纯净的氧气,可采用 (填序号)组合,其化学反应方程式为 ,确定其收集方法的依据是 .
(3)在实验室开放日,某化学兴趣小组5位同学到实验室深入探究二氧化碳的化学性质.
二氧化碳本身不能燃烧,一般也不支持燃烧,常被用做灭火剂.老师指出金属镁着火时不能用二氧化碳灭火,是由于金属镁可在二氧化碳中继续燃烧,生成白色的氧化镁和黑色的碳.小聪同学由此推理:活泼性比镁强的金属钠(Na)也能像镁一样与二氧化碳发生反应,生成的氧化钠和黑色的碳.为了证实小聪同学的推理,兴趣小组的同学在老师的指导下,完成了“钠在二氧化碳中的燃烧”探究实验.
①实验室制取二氧化碳
实验室制取和收集二氧化碳,选择上图装置中的 填序号),其反应的化学方程式为 .若要得到干燥纯净的二氧化碳,可以将发生装置产生的气体先通过装有碳酸氢钠溶液的洗气瓶除去HCl气体,再通过装有浓硫酸的洗气瓶,除去 .
②金属钠在二氧化碳中的燃烧如右图装置所示,图中氯化钯溶液用来检测是否有一氧化碳生成(一氧化碳能与氯化钯(PdCl2)反应生成黑色的钯).切取一薄片金属钠,用滤纸吸干煤油,再除去表面的氧化层放在干燥的硬质玻璃管里.实验开始时先通入干燥的二氧化碳气体,此时PdCl2溶液没明显变化,但澄清石灰水中出现 (填现象),有关反应的化学方程式为 .排尽玻璃管里的空气后,继续通入二氧化碳气体.在玻璃管底部加热,过一会,钠在充满二氧化碳气体的玻璃管里燃烧起来,产生白烟,PdCl2溶液中有黑色物质生成.实验结果表明:金属钠能与二氧化碳发生反应,但生成物中没有黑色的碳,而是一氧化碳.这跟镁与二氧化碳发生反应的结果是不一样的.金属钠与二氧化碳发生反应是否生成氧化钠呢?该兴趣小组同学讨论后认为:金属钠与二氧化碳发生反应生成的白烟可能是氧化钠,也可能是碳酸钠,或者是氧化钠和碳酸钠的混合物.已知氧化钠能与水反应:Na2O+H2O═2NaOH,反应后所得溶液呈现碱性.为了检验金属钠与二氧化碳反应生成的白烟成分,兴趣小组同学进行了如下实验,并根据实验现象得出了结论.
根据实验结果可知,金属钠与二氧化碳发生反应的化学反应方程式为 .

(1)图中有标号的仪器名称:①
(2)要在实验室中制取并收集一瓶比较纯净的氧气,可采用
(3)在实验室开放日,某化学兴趣小组5位同学到实验室深入探究二氧化碳的化学性质.
二氧化碳本身不能燃烧,一般也不支持燃烧,常被用做灭火剂.老师指出金属镁着火时不能用二氧化碳灭火,是由于金属镁可在二氧化碳中继续燃烧,生成白色的氧化镁和黑色的碳.小聪同学由此推理:活泼性比镁强的金属钠(Na)也能像镁一样与二氧化碳发生反应,生成的氧化钠和黑色的碳.为了证实小聪同学的推理,兴趣小组的同学在老师的指导下,完成了“钠在二氧化碳中的燃烧”探究实验.
①实验室制取二氧化碳
实验室制取和收集二氧化碳,选择上图装置中的
②金属钠在二氧化碳中的燃烧如右图装置所示,图中氯化钯溶液用来检测是否有一氧化碳生成(一氧化碳能与氯化钯(PdCl2)反应生成黑色的钯).切取一薄片金属钠,用滤纸吸干煤油,再除去表面的氧化层放在干燥的硬质玻璃管里.实验开始时先通入干燥的二氧化碳气体,此时PdCl2溶液没明显变化,但澄清石灰水中出现

| 实验步骤 | 实验现象 | 结论 |
| 取试管中冷却后的白色固体溶于水,分别置于两支试管中 | 滴加过量 |
白色固体不可能含有 |
滴加 |
白色固体是 |
 水是重要的资源,是人及一切生物生存所必需的,自然界的水含有各种杂质,很少能直接使用,须进行净化.
水是重要的资源,是人及一切生物生存所必需的,自然界的水含有各种杂质,很少能直接使用,须进行净化.