��Ŀ����
 ��1��ȼ�ϵ�ȼ�����������ķ�չ�����������൱��Ҫ�����ã��ӻ��������ĽǶȿ��ǣ���ú���������������У���ѡ��ȼ����
��1��ȼ�ϵ�ȼ�����������ķ�չ�����������൱��Ҫ�����ã��ӻ��������ĽǶȿ��ǣ���ú���������������У���ѡ��ȼ����H2
H2
��������������ũ������ƹ�ʹ����������������Ҫ�ɷ���CH4
CH4
��ú������Ҫ�ɷ���CO����ȼ�յĻ�ѧ����ʽΪ2CO+O2
2CO2
| ||
2CO+O2
2CO2
| ||
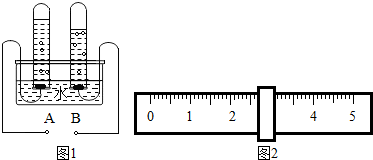
��2����ͼ1���ˮʵ���е�������ش�A��Ϊ��Դ��
��
��
����B���Թ������ɵ�����ΪH2
H2
����д����ѧʽ����3������ ��Ӳ�� ������ʯ �۸������ ��ҽ��75%�ľƾ� ����ˮ ���� �������ʣ��������ڻ������У�����ţ���ͬ��
�٢ڢܢ�
�٢ڢܢ�
�����ڴ���������ۢ�
�ۢ�
�����ڻ����������
��
�����ڵ��ʵ�����
��
����4��������ƽ������������̳�ֽ��������ͭ��ĩ�����̳�ֽ����һ��7g���룬�����߶�����ͼ2��ʾ����ʱ��ƽƽ�⣮����������ͭ����Ϊ
9.7g
9.7g
������������������̣�����ͭ��ĩ���������̣���ʵ�ʳ�������Ʒ����Ϊ4.3g
4.3g
����λͬѧ����Ͳ��ȡҺ�壬�������Ӷ���Һ�����Ϊ32mL����������Һ�����ͬѧ���Ӷ���Һ�����Ϊ24mL����Һ�����С��
��
������ڻ���С�ڡ���8mL����������1����������ȼ������ˮ����Ⱦ����������Ҫ�ɷ��Ǽ����Լ�һ����̼ȼ�����ɶ�����̼���н��
��2������ˮͨ��ֽ������������������������������������Ϊ2��1���н��
��3���������ʵķ�����н��
��4��������ƽ����Ͳ��ע��������н��
��2������ˮͨ��ֽ������������������������������������Ϊ2��1���н��
��3���������ʵķ�����н��
��4��������ƽ����Ͳ��ע��������н��
����⣺��1��ú������Ҫ�ɷ���һ����̼��ȼ�պ�����ɶ�����̼�����������ЧӦ����Ҫ���壬�����ڻ�����������������Ҫ�ɷ��Ǽ��飬ȼ�պ�Ҳ�����ɶ�����̼��������ȼ�պ�����ˮ���Ի�������Ⱦ��������ѡȼ����������������Դ�㷺����������Ҫ�ɷ���CH4��ú������Ҫ�ɷ���CO�����ݻ�ѧ����ʽ����дԭ��Ӧ��Ϊһ����̼��������������Ϊ������̼����Ӧ����Ϊ��ȼ����ȼ�յĻ�ѧ����ʽΪ��2CO+O2
2CO2�����H2��CH4��2CO+O2
2CO2��
��2��ˮͨ��ֽ������������������������������������Ϊ2��1������A��Ϊ��Դ��������B���Թ������ɵ�����Ϊ�������������H2��
��3����������ɶ���������ɵ����ʣ�����Ӳ��������ʯ��ҽ��75%�ľƾ�����ˮ�����ڻ�������������һ��������ɵ����ʣ����Ը�����ء��������ڴ������������ͬ��Ԫ����ɵĴ�������������ɲ�ͬ��Ԫ����ɵĴ�������Ը���������ڻ�������������ڵ��ʣ�����٢ڢܢݣ��ۢޣ��ۣ��ޣ�
��4������ͭ����=���������+���������=7g+2.7g=9.7g������������������̣�����ͭ��ĩ���������̣���ʵ�ʳ�������Ʒ����Ϊ7g-2.7g=4.3g�����Ӷ���Һ�����Ϊ24mL��ʵ��ʣ���Һ���������24mL��������Һ�����С��8mL�����9.7g��4.3g��С�ڣ�
| ||
| ||
��2��ˮͨ��ֽ������������������������������������Ϊ2��1������A��Ϊ��Դ��������B���Թ������ɵ�����Ϊ�������������H2��
��3����������ɶ���������ɵ����ʣ�����Ӳ��������ʯ��ҽ��75%�ľƾ�����ˮ�����ڻ�������������һ��������ɵ����ʣ����Ը�����ء��������ڴ������������ͬ��Ԫ����ɵĴ�������������ɲ�ͬ��Ԫ����ɵĴ�������Ը���������ڻ�������������ڵ��ʣ�����٢ڢܢݣ��ۢޣ��ۣ��ޣ�
��4������ͭ����=���������+���������=7g+2.7g=9.7g������������������̣�����ͭ��ĩ���������̣���ʵ�ʳ�������Ʒ����Ϊ7g-2.7g=4.3g�����Ӷ���Һ�����Ϊ24mL��ʵ��ʣ���Һ���������24mL��������Һ�����С��8mL�����9.7g��4.3g��С�ڣ�
�����������Ҫ���ջ�ѧ����ʽ����д�����ͽ��ܻ�������Ҫ�ԣ�ֻ���������ܶ���ط��������������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2005?���ϣ���1������ЧӦ�IJ���������ж�����̼�����������йأ���ͼ�п�֪�������ж�����̼�������ˡ���ֲ��ĺ�����ú��ȼ�ϵ�ȼ�գ�����Ȼ���������Ķ�����̼����
��2005?���ϣ���1������ЧӦ�IJ���������ж�����̼�����������йأ���ͼ�п�֪�������ж�����̼�������ˡ���ֲ��ĺ�����ú��ȼ�ϵ�ȼ�գ�����Ȼ���������Ķ�����̼����