��Ŀ����
ij��ȤС���ͬѧΪ�˲ⶨ̼������Ʒ��ֻ�����Ȼ��ƣ������������ʣ���̼���Ƶĺ�����ȡ�û������Ʒ������ϡ����ǡ����ȫ��Ӧ���й�ʵ�����ݼ�������
������ʵ������
��1����Ӧ���ɶ�����̼������Ϊ g��
��2����Ʒ̼���Ƶ����������Ƕ��٣��������������0.1%��
��3����Ӧ��������Һ���������������Ƕ��٣�
������ʵ������
| ��Ӧǰ | ��Ӧ�� | |||
| ʵ������ | �ձ�������/g | ϡ���������/g | �������Ʒ������/g | �ձ������л���������/g |
| 40.6 | 123.4 | 12 | 171.6 | |
��2����Ʒ̼���Ƶ����������Ƕ��٣��������������0.1%��
��3����Ӧ��������Һ���������������Ƕ��٣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����ݻ�ѧ����ʽNa2CO3+2HCl�T2NaCl+H2O+CO2������֪��Ӧǰ����ٵ������������ɶ�����̼��������
��2�����ݻ�ѧ����ʽ�����ɶ�����̼���������Լ������Ʒ��Na2CO3����������������ô�����Ʒ��Na2CO3������������
��2�����ݻ�ѧ����ʽ�����ɶ�����̼���������Լ������Ʒ��Na2CO3����������������ô�����Ʒ��Na2CO3������������
����⣺��1�����ݻ�ѧ����ʽNa2CO3+2HCl�T2NaCl+H2O+CO2�����������غ㶨�ɿ�֪��Ӧǰ����ٵ������������ɶ�����̼����������40.6g+123.4g+12g-171.6g=4.4g��
��2������Ʒ��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 117 44
x y 4.4g
=
=
x=10.6g�� y=11.7g��
��Ʒ̼���Ƶ����������ǣ�
��100%=88.3%��
��3����Ӧ��������Һ���������������ǣ�
��100%=10%��
�𣺣�1����Ӧ���ɶ�����̼������Ϊ4.4g��
��2����Ʒ̼���Ƶ�����������88.3%��
��3����Ӧ��������Һ����������������10%��
��2������Ʒ��̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 117 44
x y 4.4g
| 106 |
| x |
| 44 |
| 4.4g |
| 117 |
| y |
| 44 |
| 4.4g |
x=10.6g�� y=11.7g��
��Ʒ̼���Ƶ����������ǣ�
| 10.6g |
| 12g |
��3����Ӧ��������Һ���������������ǣ�
| 11.7g+(12g-10.6g) |
| 171.6g-40.6g |
�𣺣�1����Ӧ���ɶ�����̼������Ϊ4.4g��
��2����Ʒ̼���Ƶ�����������88.3%��
��3����Ӧ��������Һ����������������10%��
������Ҫ�����������Ŀ��Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽�衢��ʽ�Լ���֮��ص�֪ʶ�ȣ�

��ϰ��ϵ�д�
 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
�����Ŀ
���й��̣�һ��������ѧ�仯���ǣ�������
| A�����͵�� | B������ĥ���� |
| C����ʳ��� | D����¯��ը |
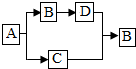 ��������A��B��C��D���dz��л�ѧ�еij������ʣ�����֮�������ͼ��ʾ��ת����ϵ����Ӧ��������Щ��Ӧ����������ȥ��������A��B�����Ԫ����ͬ��C��ʹ�����ǵ�ľ����ȼ��D��һ����ɫ���嵥�ʣ�
��������A��B��C��D���dz��л�ѧ�еij������ʣ�����֮�������ͼ��ʾ��ת����ϵ����Ӧ��������Щ��Ӧ����������ȥ��������A��B�����Ԫ����ͬ��C��ʹ�����ǵ�ľ����ȼ��D��һ����ɫ���嵥�ʣ�