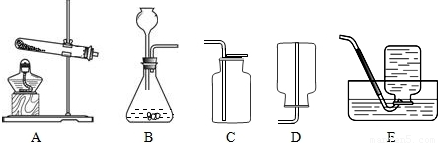
��Ŀ����
��2008?���ϣ�������ʾ����ͼ��Ϊ���������Ʊ������������ʵ��IJ���װ�ã��Ը�����ĿҪ�ش��������⣺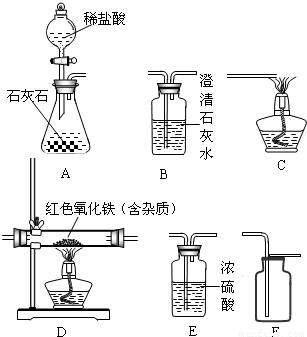
��1������ʵ�������Ʊ����ռ�����Ķ�����̼���壮
����ѡװ�õ�����˳��Ϊ______����дװ�������ĸ����
��װ��A�У�������Ӧ�Ļ�ѧ����ʽΪ______��
��2������һ����̼����ⶨij������������Ʒ�Ĵ��ȣ����ʲ���Ӧ��������֤��Ӧ����������������ʣ���ѡװ�õ�����˳��ΪD��B��C��
��װ��B�п�����������______��
��ͨ��������Ӧǰ����D�������ܵ������仯����������������Ʒ�Ĵ��ȣ���ʵ�������������е������ﻹ��������ɫ����������������Ʒ���ȵĽ����______���ƫ��ƫС������Ӱ�족������֮һ����
���𰸡���������1��ʵ�������Ʊ����ռ�����Ķ�����̼���壬���ȸ��������ҩƷ��ѡ��Ӧװ�ã�Ȼ���ɶ�����̼��������ѡȡ������������ݶ�����̼���������ʣ��ȿ�������ѡ���ռ�װ�ü�������
��2����һ����̼��������������ѧ��Ӧ�����µ����������̼������������̼ͨ������ʯ��ˮ�У����̼��Ʒ�����ѧ��Ӧ�����ɳ�������������Ĵ���=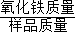 ×100%��
×100%��
����⣺��1��ʵ�������Ʊ����ռ�����Ķ�����̼���壬Ҫѡ�õ�ҩƷ��ϡ�����ʯ��ʯ�����������̼��ҩƷ��Ũ���ᣬ��Ϊ������̼�ȿ����أ�����ѡ�������ſ��������ռ����壻�����Ҫ�õ���װ���Ƿ���װ��A�����������̼����װ��E���ռ�װ��F��������˳����Ӧ������ȡ���塢�������塢�ռ����壻
��2���ٷ���װ��D��Ļ�ѧ��ӦΪ��������+һ����̼ ��+������̼�������ԣ�ͨ��װ��B�������Ƕ�����̼����
��+������̼�������ԣ�ͨ��װ��B�������Ƕ�����̼����
������̼+�������ơ�̼��ơ�+ˮ�����ԣ�װ��B�п����������dz����ʯ��ˮ������ˣ�����ʵ�������������е������ﻹ��������ɫ��������˵��������û����ȫ��Ӧ������ʣ�࣬��Ϊ�������Ĵ���=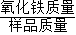 ×100%�����ԣ���ʵ�������������е������ﻹ��������ɫ����������������Ʒ���ȵĽ����ƫС��
×100%�����ԣ���ʵ�������������е������ﻹ��������ɫ����������������Ʒ���ȵĽ����ƫС��
����Ĵ��ǣ���1����A��E��F����CaCO3+2HCl=CaCl2+H2O+CO2��
��2���ٳ����ʯ��ˮ����ǣ���ƫС
�����������ۺϿ�����ʵ������ȡ������̼��װ�õ�ѡȡ������˳��һ����̼��ԭ�������Ļ�ѧ��Ӧ�Լ���ѧ����ʽ����д��
��2����һ����̼��������������ѧ��Ӧ�����µ����������̼������������̼ͨ������ʯ��ˮ�У����̼��Ʒ�����ѧ��Ӧ�����ɳ�������������Ĵ���=
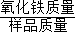 ×100%��
×100%������⣺��1��ʵ�������Ʊ����ռ�����Ķ�����̼���壬Ҫѡ�õ�ҩƷ��ϡ�����ʯ��ʯ�����������̼��ҩƷ��Ũ���ᣬ��Ϊ������̼�ȿ����أ�����ѡ�������ſ��������ռ����壻�����Ҫ�õ���װ���Ƿ���װ��A�����������̼����װ��E���ռ�װ��F��������˳����Ӧ������ȡ���塢�������塢�ռ����壻
��2���ٷ���װ��D��Ļ�ѧ��ӦΪ��������+һ����̼
 ��+������̼�������ԣ�ͨ��װ��B�������Ƕ�����̼����
��+������̼�������ԣ�ͨ��װ��B�������Ƕ�����̼����������̼+�������ơ�̼��ơ�+ˮ�����ԣ�װ��B�п����������dz����ʯ��ˮ������ˣ�����ʵ�������������е������ﻹ��������ɫ��������˵��������û����ȫ��Ӧ������ʣ�࣬��Ϊ�������Ĵ���=
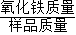 ×100%�����ԣ���ʵ�������������е������ﻹ��������ɫ����������������Ʒ���ȵĽ����ƫС��
×100%�����ԣ���ʵ�������������е������ﻹ��������ɫ����������������Ʒ���ȵĽ����ƫС������Ĵ��ǣ���1����A��E��F����CaCO3+2HCl=CaCl2+H2O+CO2��
��2���ٳ����ʯ��ˮ����ǣ���ƫС
�����������ۺϿ�����ʵ������ȡ������̼��װ�õ�ѡȡ������˳��һ����̼��ԭ�������Ļ�ѧ��Ӧ�Լ���ѧ����ʽ����д��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ