��Ŀ����
��6�֣�ͬѧ������ij������Һ�����������ϣ���Ҫ��������ʱ���۲쵽��Һ���̲��ŵ��̼�����ζ�����ַ�����������ͬѧ�ǵ�˼������Ԫ����ɵĽǶȷ��������Dz²����������SO2��CO��CO2��H2�е�һ�ֻ��֡�

��1����ͬѧ��Ϊ������һ����SO2��ԭ���� ��
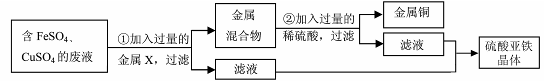
��2��Ϊ��ȷ��������ijɷ֣�ͬѧ�������һ������ʵ��װ�ã�����ͼ��ʾ������̨����ȥ��������������Լ���ѡ��ҩƷ�����ʵ��װ�����ʵ�顣
ҩƷ������ʯ��ˮ�����Ը��������Һ����ˮ����ͭ����ˮ����������ʯ�ң�����ˮ��CO2������ҩƷ���ظ�ѡ�ã�ÿһ������Ӧ��֡�

����B��ʢ�ŵ��Լ��� ��A��Bװ�ò��ܻ�����ԭ���ǣ� ��
�������۲쵽 ����֤��ԭ��������CO��H2��д��D�з�Ӧ�Ļ�ѧ����ʽ ��
����D�з�Ӧǰ����Ʒ10g����Ʒ�п��ܻ�����������ȫ��Ӧ��ʣ�����7.6g����ԭ��Ʒ�ijɷ�Ϊ�� ����д�����ʼ����Ӧ��������

��1����ͬѧ��Ϊ������һ����SO2��ԭ���� ��
��2��Ϊ��ȷ��������ijɷ֣�ͬѧ�������һ������ʵ��װ�ã�����ͼ��ʾ������̨����ȥ��������������Լ���ѡ��ҩƷ�����ʵ��װ�����ʵ�顣
ҩƷ������ʯ��ˮ�����Ը��������Һ����ˮ����ͭ����ˮ����������ʯ�ң�����ˮ��CO2������ҩƷ���ظ�ѡ�ã�ÿһ������Ӧ��֡�

����B��ʢ�ŵ��Լ��� ��A��Bװ�ò��ܻ�����ԭ���ǣ� ��
�������۲쵽 ����֤��ԭ��������CO��H2��д��D�з�Ӧ�Ļ�ѧ����ʽ ��
����D�з�Ӧǰ����Ʒ10g����Ʒ�п��ܻ�����������ȫ��Ӧ��ʣ�����7.6g����ԭ��Ʒ�ijɷ�Ϊ�� ����д�����ʼ����Ӧ��������
��1������������ֻ��SO2�д̼�����ζ
��2��������ʯ��ˮ SO2Ҳ��ʹ����ʯ��ˮ����ǣ����ж��Ƿ���CO2
����D�й����ɺ��ڣ�E�������Ա仯��F�г���ʯ��ˮ�����
3CO + Fe2O3 2Fe + 3CO2
2Fe + 3CO2
������������8g ����2g
��2��������ʯ��ˮ SO2Ҳ��ʹ����ʯ��ˮ����ǣ����ж��Ƿ���CO2
����D�й����ɺ��ڣ�E�������Ա仯��F�г���ʯ��ˮ�����
3CO + Fe2O3
 2Fe + 3CO2
2Fe + 3CO2 ������������8g ����2g
�����������1����ͬѧ��Ϊ������һ����SO2��ԭ��������������ֻ��SO2�д̼�����ζ��
��2������A��ʢ�����Ը��������Һ��B��ʢ�ŵ��Լ��dz���ʯ��ˮ��C��ʢ�ż�ʯ�ң�E��ʢ����ˮ����ͭ��F��ʢ�ų���ʯ��ˮ��A��Bװ�ò��ܻ�����ԭ���ǣ�SO2Ҳ��ʹ����ʯ��ˮ����ǣ����ж��Ƿ���CO2��
�������۲쵽D�й����ɺ��ڣ�E�������Ա仯��F�г���ʯ��ˮ����ǵ�����֤��ԭ��������CO��H2��D�з�Ӧ�Ļ�ѧ����ʽΪ3CO + Fe2O3
 2Fe + 3CO2 ��
2Fe + 3CO2 ������D�з�Ӧǰ����Ʒ10g����Ʒ�п��ܻ�����������ȫ��Ӧ��ʣ�����7.6g������Ʒ�к�����Ԫ��10g-7.6g=2.4g����������������ΪX����X��
 =2.4g��X=8g������Ʒ�к���8g��������10g-8g=2g����
=2.4g��X=8g������Ʒ�к���8g��������10g-8g=2g������������������ʱ��Ҫ�������ʵ����ʣ�����ʵ�飬ͨ����ͬ��ʵ��������з������ų����ţ�
������̼��ʹ����ʯ��ˮ����ǣ�����������ʱ���Ը��������Һ��ɫ��
ijԪ�ص���������=
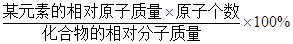 ��
��
��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ