��Ŀ����
��5�֣���Դ��������������������ᷢչ������ء�
��1��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ�������Ļ�ʯȼ�ϰ���ú��ʯ�ͺ� ��
��2��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ�����壬�˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ������ʾ��
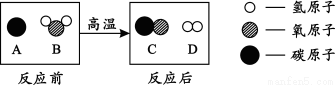
�� �÷�Ӧ�Ļ�ѧ����ʽΪ ��
�� �÷�Ӧ������ķ��Ӹ�����Ϊ ��
��3��Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϡ����о�����NH3ȼ�յIJ���û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����
�� NH3�е�Ԫ�غ���Ԫ�ص�������Ϊ ��
�� ��NH3ȼ�շ�Ӧ�Ļ�ѧ����ʽ����������4NH3 + 3O2 ��ȼ 6H2O + ��
��1����Ȼ�� ��2���� C + H2O ���� CO + H2 �� CO��H2�ķ��Ӹ�����Ϊ1��1
��3���� 14��3 �� 2N2
��������
�����������1��������ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ��
��2��������ʾ��ͼ���ɷֱ�д�������ʵĻ�ѧʽ��AΪC��BΪH2O��CΪCO��DΪH2�������� �÷�Ӧ�Ļ�ѧ����ʽΪ��C + H2O ���� CO + H2�������ݷ�Ӧ�Ļ�ѧ����ʽ�����жϸ÷�Ӧ������ķ��Ӹ�����Ϊ1��1
��3�������ݻ�ѧʽ��NH3�е�Ԫ�غ���Ԫ�ص�������=14��1��1��3=14��3
�����������غ㶨�ɵ��۽��ͣ���ѧ��Ӧǰ��ԭ�ӵ����࣬���������䣬���Բ����Ƴ���ѧ����ʽ��������Ϊ��4NH3 + 3O2 ��ȼ 6H2O + 2N2
���㣺��ʾ��ͼ�����ݻ�ѧʽ���㣬�����غ㶨�ɵ��۽���









