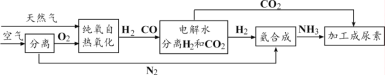
��Ŀ����
����Ŀ����ѧ������������أ�ѧ�û�ѧ���������Ƿ�����������������е����⡣��������ͭ�����������г����Ľ������밴Ҫ��ش��������⣺
��1���������Ƴ�����������������______�ԡ�
��2�����������⣬ͨ���������ɲ����ָ����Է�ֹ���⡣���г���֧��ˢ�����ᣬ�Է����⣬����Ϊ�������ʹ��������е�______��______������
��3���ڸ����£����ý�̿��������Ӧ���ɵ�һ����̼�ɰ����ӳ�����ʯ�ﻹԭ�������û�ѧ����ʽ��ʾ��������Ӧ��ԭ��______��
��4����ͭ��ͭп�Ͻ���Ӳ�ȱȴ�ͭ______��������������С������
��5��������Ͷ�뺬��Cu(NO3)2��AgNO3�Ļ����Һ�У���ַ�Ӧ����ˣ�����Һ��ֻ��һ�����ʣ�����������______�����˺��������һ������______�����ܺ���______��
���𰸡���չ ���� ˮ Fe2O3+3CO![]() 2Fe+3CO2 �� �������� ͭ���� ��
2Fe+3CO2 �� �������� ͭ���� ��
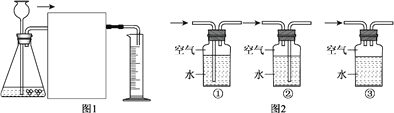
��������
��1�����ý������������õ���չ�Կɰѽ������Ƴ�Ƭ���ʴ�Ϊ����չ��
��2��������������������ˮͬʱ�Ӵ������г���֧��ˢ���������Է��⣬����Ϊ�������ʹ��������е�������ˮ���������������ˮ��
��3���ڸ����£���������һ����̼��Ӧ�Ļ�ѧ����ʽΪ��Fe2O3+3CO![]() 2Fe+3CO2�����Fe2O3+3CO
2Fe+3CO2�����Fe2O3+3CO![]() 2Fe+3CO2��
2Fe+3CO2��
��4�����ںϽ��Ӳ�ȱ�������Ĵ�������Ӳ�ȴ������ͭп��ɵĻ�ͭ�Ͻ��Ӳ�ȱȴ�ͭ��Ӳ�ȴ����
��5���������˳���У�����ǰ��Ľ����ܽ����ں���Ľ������仯�����ˮ��Һ���û��������ҽ�����Բ��Խ��Խ��Ӧ�����ȷ�����Ӧ�� Fe+2AgNO3=2Ag+ Fe(NO3)2����AgNO3��Ӧ���Ժ�����Cu(NO3)2��Һ��Ӧ��Fe+Cu(NO3)2= Fe(NO3)2 + Cu����Һ��ֻ��һ�����ʣ���Ϊ��������������ͭ�Ѿ�����ȫ�û���������������ʣ�࣮
�ʴ�Ϊ������������ͭ����������

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�����Ŀ����ʵ�����л�ѧ��ȤС���ͬѧ������һƿ���ڷ��õ�Ca(OH)2��ĩ״�Լ������ǶԸ��Լ������˶���ʵ���ȡ�Լ�19.8 g������ƿ�У�����30.2 g��ˮ�������������ƿ�����εμ�25 g�����ַ�Ӧ����ò���������ͼ�����¡�������й���Ϣ�ش����⡣
��1�� | ��2�� | ��3�� | ��4�� | ��5�� | ��6�� | |
�������������/g | 25 | 25 | 25 | 25 | 25 | 25 |
��ƿ�����ʵ�����/g | 75 | 100 | a | 150 | 172.8 | 197.8 |
(1)a����ֵΪ__________����ȤС���ͬѧͨ��ʵ�飬�ó����Լ��Ѳ��ֱ��ʵĽ��ۣ���������(ͨ��ʵ�����ݺ�ͼ��д����ķ���) _______________________________��
(2)b����ֵΪ____________��
(3)������Լ����������Ƶ���������___________(д��������̣��������һλС��)��