��Ŀ����
(12��)�밴Ҫ�����
С��1:��д����ԵĻ�ѧ���Ż����ƣ�
��+2�۵�ͭԪ��______ ��2NO3 ____ ��2��������______ ��3����ԭ��
____ ��2��������______ ��3����ԭ��
С��2: ���H��N��O��Ca����Ԫ����ѡ��ǡ����Ԫ�أ�д����������Ҫ�����ʵĻ�ѧʽ��
�ٸ������������ļ�___________ ����ʹʪ���ɫʯ����ֽ����������________
�����������������������_______ �ܳ�����ʳƷ�����������______________
С��3:д����������Ҫ��Ļ�ѧ����ʽ��
����ɫֲ����й������__________�� ����Al(OH)3����θ�����__________��
��������ͭʪ����ͭ �� �ܼ���ȼ�����ɶ�����̼��ˮ___________��
С��1:��д����ԵĻ�ѧ���Ż����ƣ�
��+2�۵�ͭԪ��______ ��2NO3
 ____ ��2��������______ ��3����ԭ��
____ ��2��������______ ��3����ԭ��С��2: ���H��N��O��Ca����Ԫ����ѡ��ǡ����Ԫ�أ�д����������Ҫ�����ʵĻ�ѧʽ��
�ٸ������������ļ�___________ ����ʹʪ���ɫʯ����ֽ����������________
�����������������������_______ �ܳ�����ʳƷ�����������______________
С��3:д����������Ҫ��Ļ�ѧ����ʽ��
����ɫֲ����й������__________�� ����Al(OH)3����θ�����__________��
��������ͭʪ����ͭ �� �ܼ���ȼ�����ɶ�����̼��ˮ___________��
��1:
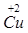 2����������� 2O2 3Ag
2����������� 2O2 3Ag��1: Ca(OH)2 NH3 NO2 CaO
��1: 6CO2 + 6H2O
 C6H12O6 + 6O2
C6H12O6 + 6O2Al(OH)3 + 3HCl = AlCl3 + 3H2O
Fe + CuSO4 = FeSO4 + Cu
CH4 + 2O2
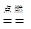 CO2 + 2H2O
CO2 + 2H2O�⣺��1���ٻ��ϼ۵ı�ʾ�������ڸ�Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں����ͭԪ�صĻ��ϼ�Ϊ+2��ʾΪ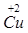 ��
��
��2NO3-����2��ʾ�������ĸ�����NO3-��ʾ������������ӣ�����2NO3-��ʾ����2����������ӣ�
���������ӵķ����������Ļ�ѧʽ������������ڻ�ѧʽǰ�����ϵ��2������2�������ӵķ���Ϊ��2O2��
����ԭ�ӵķ�������Ԫ�صķ��ţ�����������ڻ�ѧʽǰ�����ϵ��3������3����ԭ�ӵķ���Ϊ��3Ag��
��2���ٸ������������ļ����������ƣ��仯ѧʽΪ��Ca��OH��2��
����ʹʪ���ɫʯ����ֽ��������������Һ�Լ��ԣ�����Ԫ�ص�������ж��仯ѧʽΪ NH3��
�����������������������Ϊ������������ѧʽΪNO2��
�ܳ�����ʳƷ����������ʺܶ࣬��Ũ���ᡢ��ʯ�ҵȣ�����Ԫ�صķ�Χ��֪�����Ʒ���Ҫ���仯ѧʽΪ��CaO��
��3������ɫֲ����й��������ֲ�ォ������̼��ˮת��Ϊ�����Ǻ���������ѧ����ʽΪ��6CO2 + 6H2O C6H12O6 + 6O2
C6H12O6 + 6O2
����Al(OH)3����θ����࣬�����������������к�θ�������κ�ˮ����ѧ����ʽΪ��Al(OH)3 + 3HCl = AlCl3 + 3H2O
��������ͭʪ����ͭ����������Һ�н������û���Ӧ����ѧ����ʽΪ��Fe + CuSO4 = FeSO4 + Cu
�ܼ���ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪ��CH4 + 2O2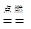 CO2 + 2H2O
CO2 + 2H2O
�ʴ�Ϊ��С��1: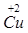 2����������� 2O2 3Ag
2����������� 2O2 3Ag
��1: Ca(OH)2 NH3 NO2 CaO
��1: 6CO2 + 6H2O C6H12O6 + 6O2
C6H12O6 + 6O2
Al(OH)3 + 3HCl = AlCl3 + 3H2O
Fe + CuSO4 = FeSO4 + Cu
CH4 + 2O2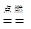 CO2 + 2H2O
CO2 + 2H2O
������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д����Ӧ����ʽ����д����������֪ʶ�����⣻
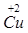 ��
����2NO3-����2��ʾ�������ĸ�����NO3-��ʾ������������ӣ�����2NO3-��ʾ����2����������ӣ�
���������ӵķ����������Ļ�ѧʽ������������ڻ�ѧʽǰ�����ϵ��2������2�������ӵķ���Ϊ��2O2��
����ԭ�ӵķ�������Ԫ�صķ��ţ�����������ڻ�ѧʽǰ�����ϵ��3������3����ԭ�ӵķ���Ϊ��3Ag��
��2���ٸ������������ļ����������ƣ��仯ѧʽΪ��Ca��OH��2��
����ʹʪ���ɫʯ����ֽ��������������Һ�Լ��ԣ�����Ԫ�ص�������ж��仯ѧʽΪ NH3��
�����������������������Ϊ������������ѧʽΪNO2��
�ܳ�����ʳƷ����������ʺܶ࣬��Ũ���ᡢ��ʯ�ҵȣ�����Ԫ�صķ�Χ��֪�����Ʒ���Ҫ���仯ѧʽΪ��CaO��
��3������ɫֲ����й��������ֲ�ォ������̼��ˮת��Ϊ�����Ǻ���������ѧ����ʽΪ��6CO2 + 6H2O
 C6H12O6 + 6O2
C6H12O6 + 6O2����Al(OH)3����θ����࣬�����������������к�θ�������κ�ˮ����ѧ����ʽΪ��Al(OH)3 + 3HCl = AlCl3 + 3H2O
��������ͭʪ����ͭ����������Һ�н������û���Ӧ����ѧ����ʽΪ��Fe + CuSO4 = FeSO4 + Cu
�ܼ���ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪ��CH4 + 2O2
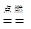 CO2 + 2H2O
CO2 + 2H2O�ʴ�Ϊ��С��1:
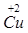 2����������� 2O2 3Ag
2����������� 2O2 3Ag��1: Ca(OH)2 NH3 NO2 CaO
��1: 6CO2 + 6H2O
 C6H12O6 + 6O2
C6H12O6 + 6O2Al(OH)3 + 3HCl = AlCl3 + 3H2O
Fe + CuSO4 = FeSO4 + Cu
CH4 + 2O2
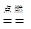 CO2 + 2H2O
CO2 + 2H2O������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д����Ӧ����ʽ����д����������֪ʶ�����⣻

��ϰ��ϵ�д�
�����Ŀ