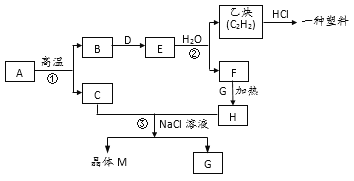
��Ŀ����
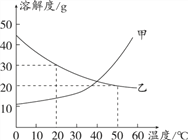
����Ŀ����ͼ�� NH4Cl��NaCl�� NaHCO3�������ʵ��ܽ�����ߡ�

��1������������______________������������_____________��
��2��30��ʱ��NaHCO3���ܽ����_________�����¶��£���100gˮ�м���40 gNaHCO3��������Һ����������Һ��������Ϊ__________
��3������˵����ȷ����_______________��
A.NH4Cl������Һһ���Ȳ�������ҺŨ
B.����NaCl��Һ�����������Һ�����ʵ������������ܲ���
C.30�棬�ֱ�10 gNaCl��10gNH4Cl���Ƴɱ�����Һ��ǰ����Ҫˮ�������϶�
D.40�棬�ֱ�NaCl��NH4Cl�ı�����Һ���µ�20��ʱ�����������ľ���϶�
���𰸡� NaC1��NH4C1�� NaHCO3 10g�� 1:11 BC
����������1�����ݳ������ܽ�ȴ���10g���������ʣ��ܽ����1--10֮���ǿ������ʽ����2�������ܽ�����߷�����𣻸��ݼ�������3��A�������¶Ȳ�ȷ�����������B�����ݺ��������ܼ����C������30��ʱNaCl��NH4Cl���ܽ�ȷ������D��������Һ���������������1����20��ʱ���Ȼ��ƺ��Ȼ�淋��ܽ�ȴ���10g�����������ʣ�̼�����Ƶ��ܽ�ȴ���1С��10���ǿ�����������2�����ܽ�����߿�֪��30��ʱ��NaHCO3���ܽ����10g�����¶��£���100gˮ�м���40gNaHCO3��ֻ���ܽ�10gNaHCO3����������Һ����������Һ��������Ϊ10g����10g+100g��=1��11����3��A���¶Ȳ�ȷ�������Ƚϣ�������B�������ú��������ܼ��ķ���������NaCl��Һ�����������Һ�����ʵ������������䣬��ȷ��C��30��ʱNaCl���ܽ��С��NH4Cl���ܽ�ȣ��ʽ�10gNaCl��10gNH4Cl���Ƴɱ�����Һ��ǰ����Ҫˮ�������϶࣬��ȷ��D����Һ������ȷ�������жϣ�����ѡBC��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�