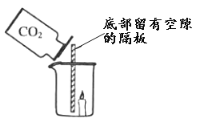
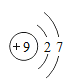��Ŀ����
����Ŀ����ѧʵ���ǽ��п�ѧ̽������Ҫ��ʽ��
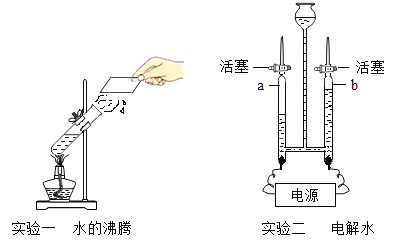
��1������ʵ�������в������ڼ��ȵ���________������ĸ���ţ���
a���ձ� b����Ͳ c���Թ� d��������
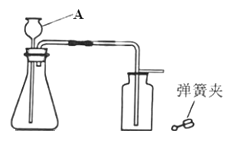
��2��ij��ѧС��ѡ������װ�ú�ҩƷ����̽��ʵ�飮
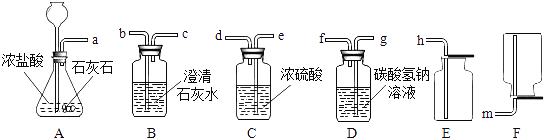
��A�з�Ӧ�Ļ�ѧ����ʽΪ__________________________
������A��B���ӣ�A�в��������岢����ʹB�е���Һ����ǣ�����Ϊʲô��____________
����Ҫ��ȡһƿ��Ϊ�����������CO2����ѡ��װ�õĵ��ܽӿڴ����ҵ���ȷ����˳��Ϊ��a��________��________��________��________��________��
��3��Ϊ̽����ҵ��ˮ���ۺ����ã�ij��ѧС����ʵ���������������ʵ�飮
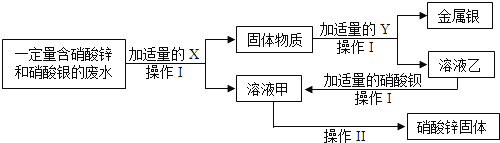
��XΪ________���������ᱵ��Ӧ�Ļ�ѧ����ʽΪ________��
�ڲ���I�Ͳ������ж�Ҫ�õ�һ�ֲ����������������ڲ������е�������ʲô��_______
��4��ij�������Ϊ����̽���÷����������ȡ�����Ʒ�����з�����4.9t��H2SO4����������Ϊ20%��������������м��Ӧ����ȡ����������ͬʱ�����ɵ�ȫ������ͨ����������ͭ�в����ȣ�H2+CuO![]() Cu+H2O�����������������������ͭ��������_______
Cu+H2O�����������������������ͭ��������_______
���𰸡�b CaCO3+2HCl�TCaCl2+CO2��+H2O Ũ�����ӷ���ʹCO2�л���HCl����������CaCO3���� g f e d h п����Zn�� ZnSO4+Ba��NO3��2=BaSO4��+Zn��NO3��2 �ò���������������Ƿ�ֹ��ֲ��¶ȹ������Һ�ηɽ� 0.64t
��������
��1���ձ��ڼ���ʱ��Ҫ����ʯ�������Թܺ��������ֱ�Ӽ��ȣ���Ͳ���ܼ��ȣ�
��2����ʯ��ʯ�е�̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��
��Ũ������лӷ��ԣ�������ȡ�Ķ�����̼�л����Ȼ������壬�Ȼ��������������Ʒ�Ӧ��
������̼��������Һ��ȥ������̼�л��е��Ȼ������壬����Ũ�����ȥ������̼��ˮ����������������ſ��������ռ���ע������ij����̳���
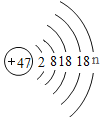
��3��������ͼ�����������X�ǹ�����п�����˺���������п�����Ļ�������ϡ���Ὣ����룬��Һ�Ҿ�������п��Һ������п�����ᱵ��Ӧ�������ᱵ��ɫ����������п���������Ǵ���Һ�л�ȡ���壬�������ᾧ���������ڴ˹����е��������ò���������������Ƿ�ֹ��ֲ��¶ȹ������Һ�ηɽ���
��4��������������ʽ��Fe+H2SO4�TFeSO4+H2����H2+CuO![]() Cu+H2O�����������ᡢ������֮ͭ���������ϵ��Ȼ����������������������ͭ���������ɡ�
Cu+H2O�����������ᡢ������֮ͭ���������ϵ��Ȼ����������������������ͭ���������ɡ�
����������������ͭ������Ϊx��
��Ϊ��Fe+H2SO4�TFeSO4+H2����H2+CuO![]() Cu+H2O���ɵù�ϵʽ��
Cu+H2O���ɵù�ϵʽ��
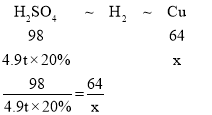
x=0.64t
�ʴ�Ϊ��
��1��b��
��2����CaCO3+2HCl�TCaCl2+CO2��+H2O����Ũ�����ӷ���ʹCO2�л���HCl����������CaCO3��������g��f��e��d��h��
��3�������Zn����ZnSO4+Ba��NO3��2=BaSO4��+Zn��NO3��2��
������ʱ�ò��������Ͻ��裬��ֹ��ֲ��¶ȹ������Һ�ηɽ���
��4��0.64t��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�