��Ŀ����
�ҹ��Ͷ������ں�����ǰ����ұ����ͭ����ͭ��ͭ��п�ĺϽ𣬿������������������DZ�������Ʒ�ȣ�ijͬѧΪ�ⶨ��ͭ��Ʒ��ͭ����������������������ʵ�飺��ȡ8g�û�ͭ��Ʒ���Ѽӳɷ�ĩ��������һ�Թ��У�Ȼ�����Թ�����ε���δ֪����������ϡ���ᣬ��ǡ��������ų�Ϊֹ����ò�������0.1g��
��1��ͨ����������û�ͭ��Ʒ��ͭ������������________
��2�����������ϡ���������ʵ���������������Ϊ����Ҫ��Щ���ݣ�________��
�⣺��1����8g��ͭ�к�п����Ϊx��
Zn+H2SO4�TZnSO4+H2��
65 2
x 0.1g
x=3.25g
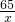 =
=
x=3.25g
Cu%= ��100%=59.4%
��100%=59.4%
�𣺻�ͭ��Ʒ��ͭ������������59.4%��
�ʴ�Ϊ��59.4%
��2���������������������������������������������ϡ���������ʵ���������������Ҫ֪����ȥϡ��������������Ա����Ϊ��ϡ�����������
��������ͭ���������ᷴӦ����п������������������������������п���������Ӷ����ͭ�ĺ���������������������������������������Ҫ���������������������Ҫ֪������ϡ�����������
���������⿼���˻�Ͻ����н��������IJⶨ����ɴ��⣬�������ݽ�������ķ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽ���У�
Zn+H2SO4�TZnSO4+H2��
65 2
x 0.1g
x=3.25g
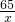 =
=
x=3.25g
Cu%=
 ��100%=59.4%
��100%=59.4%�𣺻�ͭ��Ʒ��ͭ������������59.4%��
�ʴ�Ϊ��59.4%
��2���������������������������������������������ϡ���������ʵ���������������Ҫ֪����ȥϡ��������������Ա����Ϊ��ϡ�����������
��������ͭ���������ᷴӦ����п������������������������������п���������Ӷ����ͭ�ĺ���������������������������������������Ҫ���������������������Ҫ֪������ϡ�����������
���������⿼���˻�Ͻ����н��������IJⶨ����ɴ��⣬�������ݽ�������ķ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽ���У�

��ϰ��ϵ�д�
�����Ŀ