��Ŀ����
�����������ᷢչ�벻����ѧ��
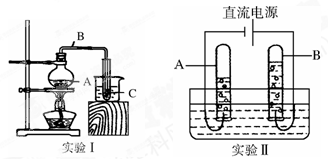
������ʳ���У����ṩ��������_________������ĸ����



A B C
������������벻�������������к�������������______���ѧʽ�������������������������Ҫ���ʣ�����������������Һ���ն�������Ļ�ѧ����ʽ��______________��
�����������벻��ˮ����ˮ�ľ��������м���������������_______�����������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿��________���á�
����ũ�ҷ���ȣ�������Ч�ɷֶ࣬��Ч�죬����ʹ�û��ʿ�һ���̶��ϴٽ���ʳ��ũ��Ʒ��������ľ�ҵ���Ҫ�ɷ���̼��أ���ˮ��Һ��______�������ԡ����ԡ����ԣ�������̼���Ƶ����ʺ����ƣ��������ǵķ�����_________________________��������ͽ��ۣ���
�������з�����δ���ҳ�ͬ�ⳤ�ڸ����ӷ�������������Ƭ(�׳Ʋ�����)�Ĵ���ҩ���仯ѧ����:N-N-(2-�һ�-���ǰ���)-���������Σ���ѧʽ��C6H13N5O��HCl������_____��Ԫ����ɣ�Ħ������Ϊ__________________��0��1mol���������Һ���______________����ԭ�ӡ�
������ʳ���У����ṩ��������_________������ĸ����



A B C
������������벻�������������к�������������______���ѧʽ�������������������������Ҫ���ʣ�����������������Һ���ն�������Ļ�ѧ����ʽ��______________��
�����������벻��ˮ����ˮ�ľ��������м���������������_______�����������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿��________���á�
����ũ�ҷ���ȣ�������Ч�ɷֶ࣬��Ч�죬����ʹ�û��ʿ�һ���̶��ϴٽ���ʳ��ũ��Ʒ��������ľ�ҵ���Ҫ�ɷ���̼��أ���ˮ��Һ��______�������ԡ����ԡ����ԣ�������̼���Ƶ����ʺ����ƣ��������ǵķ�����_________________________��������ͽ��ۣ���
�������з�����δ���ҳ�ͬ�ⳤ�ڸ����ӷ�������������Ƭ(�׳Ʋ�����)�Ĵ���ҩ���仯ѧ����:N-N-(2-�һ�-���ǰ���)-���������Σ���ѧʽ��C6H13N5O��HCl������_____��Ԫ����ɣ�Ħ������Ϊ__________________��0��1mol���������Һ���______________����ԭ�ӡ�
��BC
��N2 2NaOH + SO2=Na2SO3 + H2O
��������ɱ�� ����
�ܼ��� ��ɫ��Ӧ������ʻ�ɫ��̼���ƣ�����ɫ�ܲ���Ƭ������ɫ��̼���
��5 142��5g/mol 3��01��1023
��N2 2NaOH + SO2=Na2SO3 + H2O
��������ɱ�� ����
�ܼ��� ��ɫ��Ӧ������ʻ�ɫ��̼���ƣ�����ɫ�ܲ���Ƭ������ɫ��̼���
��5 142��5g/mol 3��01��1023
������������������ܹ��ṩ������Ӫ�������ǵ����ʡ����ࡢ��֬��A�����ʵ��߲ˡ�ˮ�����ḻ��ά���أ�B��ţ���к��зḻ�ĵ����ʣ�C��������Ҫ�ɷ��ǵ��ۣ��������࣬��ѡB��C
�ڿ����к������������ǵ�������ѧʽΪ��N2�����������������������Ҫ���ʣ�����������������Һ���ն�������Ļ�ѧ����ʽ�ǣ�2NaOH + SO2=Na2SO3 + H2O
�����������벻��ˮ����ˮ�ľ��������м��������������ǣ�������ɱ��������̿���н�ǿ�������ԣ�������ˮ�ľ����ȣ��������������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿����������
�ܲ�ľ�ҵ���Ҫ�ɷ���̼��أ���ˮ��Һ�Լ��ԣ���Ϊ����̼���Ƶ����ʺ����ƣ�ˮ��ҺҲ�Լ��ԣ�������̼�������Ҫ����������ͨ����ɫ��Ӧ�������ǣ���ɫ��Ӧ������ʻ�ɫ��̼���ƣ�����ɫ�ܲ���Ƭ������ɫ��̼���
�ݸ��ݻ�ѧʽ��C6H13N5O��HCl�����ж���5��Ԫ����ɣ�Ħ������Ϊ142��5g/mol������1mol������������5��6��02��1023����ԭ�ӣ�����0��1mol���������Һ���3��01��1023����ԭ��

��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
�����Ŀ