��Ŀ����
����Ŀ������ͼ�ش��������⡣
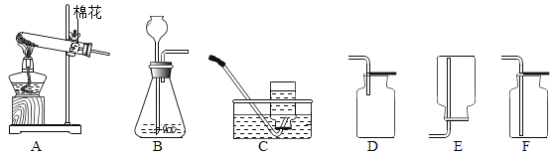
��1��д�����Тٵ���������_____��
��2��д��ʵ������Aװ����ȡ�����Ļ�ѧ��Ӧ����ʽ_____��
��3��ijͬѧҪ��ȡ������̼Ӧѡ�õ�װ�����Ϊ_____�����Ҫѡ��Fװ���ռ�����Ӧ�ô�_____��ͨ�룬���������_____��Bװ���г���©���¶�Ҫ�γ�Һ�⣬Ŀ����_____��ʵ������и�ͬѧ�۲쵽B�з�Ӧ̫�죬�����������ռ�������ѡ�����д�ʩ�е�_____������ţ���������⡣
A��������©������ע����
B����װ���м�����������ˮ
C������ƿ�����ԹܼӴ�������Ƭ
��4����Gͼ��ʾ����һƿ������̼���������������������ձ��У�����Ϊ_____�������ֶ�����̼������Ϊ��_____��_____��
���𰸡��Թܣ� 2KClO3![]() 2KCl+3O2���� BE�� a�� ��ȼ�ŵ�ľ������b���ܿڣ�ľ��Ϩ��֤�������� ��ֹ���ɵ������ݳ��� AB�� ����������������Ϩ�� ������̼�ܶȱȿ����� ����ȼ������ȼ��
2KCl+3O2���� BE�� a�� ��ȼ�ŵ�ľ������b���ܿڣ�ľ��Ϩ��֤�������� ��ֹ���ɵ������ݳ��� AB�� ����������������Ϩ�� ������̼�ܶȱȿ����� ����ȼ������ȼ��
��������
��1��ͨ������������ָ���������ƺ����ÿ�֪�������Թܣ�
��2��������ڶ������̵Ĵ������¼��������Ȼ��غ���������ѧ����ʽΪ��2KClO3![]() 2KCl+3O2����
2KCl+3O2����
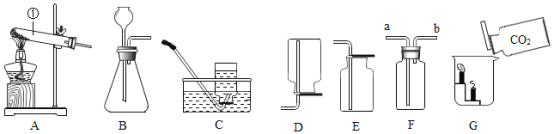
��3��ʵ������ȡ������̼�ķ�Ӧ���ǹ����Һ�壬��Ӧ�����dz��£�������̼�ܶȱȿ���������ˮ������Ӧѡ�õ�װ�����ΪBE��Ҫѡ��Fװ���ռ���Ӧ�ô�a��ͨ�룬������̼��������ȼ�ԣ������ķ����ǣ���ȼ�ŵ�ľ������b���ܿڣ�ľ��Ϩ��֤��������Bװ���г���©���¶�Ҫ�γ�Һ�⣬Ŀ���ǣ���ֹ���ɵ������ݳ���ʵ������и�ͬѧ�۲쵽B�з�Ӧ̫�죬�����������ռ������������ѡ��Ĵ�ʩ�ǣ�������©������ע��������װ���м�����������ˮ����ѡ��AB��
��4����һƿ������̼�������������ȼ��������ձ��У�����Ϊ������������������Ϩ�𣬿����ֶ�����̼������Ϊ��������̼�ܶȱȿ�������ȼ������ȼ��

����Ŀ��Ϊ�˼��ʯ��ʯ��Ʒ��̼��Ƶĺ������ס��ҡ���������λͬѧ�ֱ�������������ͬ����������Ʒ��ַ�Ӧ����ʵ��ⶨ����֪����Ʒ�е����ʲ�����ˮ���Ҳ������ᷴӦ������������±���
�� | �� | �� | �� | |
��ȡʯ��ʯ��Ʒ������g | 10.0 | 10.0 | 10.0 | 10.0 |
���������������g | 20.0 | 30.0 | 45.0 | 50.0 |
ʣ������������g | 6.0 | 4.0 | 2.0 | 2.0 |
��ش�
�� ��Ʒ��̼��Ƶ�����������_______��
��10.0 g��Ʒ��45.0 g�����ַ�Ӧ�������Ƿ���ʣ��_______(����������������)��
��10.0 g��Ʒ������ϡ���ᷴӦ��ɲ���������̼_____��?(д��������̣���������ȷ��0.1 g)
(�����õ������ԭ��������H��1 C��12 O��16 Cl��35.5 Ca��40)
����Ŀ�����ּ�ͥ������������Ϣ���±���ʾ:
���� |
|
|
|
���� | ����� | ��ƯҺ | Ư��ˮ |
��Ч�ɷ� | HCl | H2O2 | NaClO ���������ƣ� |
��1�����������ڜ[��ˮ������Ҫ�ɷ�ΪCaCO3�����÷�Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ʹ�ò�ƯҺʱ��������ֽ��ͷų�����������Ӧ�Ļ�ѧ����ʽΪ______________��
��3��������Ư��ˮ��Ϻ�HCI��NaCl0�ᷢ����Ӧ�������ж�����������Cl2����ͬʱ����ˮ��������Ԫ����ɵ��Σ�������______________��