��Ŀ����
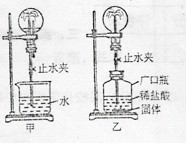
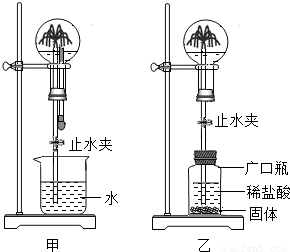
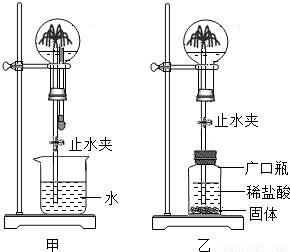
С�������ͼ��ʾװ�ý���Ȥζʵ�顣
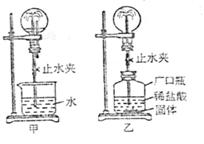 ��1��ʹ�ü�װ�ý���ʵ��ʱ�����ι��е����ʼ����ձ��У�Ȼ���ֹˮ�У����Թ۲쵽��ƿ�ڲ�����������Ȫ��
��1��ʹ�ü�װ�ý���ʵ��ʱ�����ι��е����ʼ����ձ��У�Ȼ���ֹˮ�У����Թ۲쵽��ƿ�ڲ�����������Ȫ��
a.����ƿ��ι��е����ʷ�����ѧ��Ӧ�����Ʋ�
| ��ƿ�е����� | ||
| �ι��е����� |
b.����ƿ��ι��е����ʲ�������ѧ��Ӧ�����Ʋ�
| ��ƿ�е����� | |
| �ι��е����� |
��2��ʹ����װ�ý���ʵ��ʱ��Ҳͬ���γ�����Ȫ��С��ͬѧ����Ĺ������ʲ������� �� A��п�� B��̼��� C�������� D��þ��
д�����������Ļ�ѧ����ʽ 
��3�����г�������Ȫ�Լ���Ȼ���еĻ�ɽ����ԭ�������� ����ס����ҡ���װ��ԭ����ͬ��
��1��a CO2 (SO2)��ŨNaOH��KOH����Һ��HCl��NaOH��K OH����Һ
OH����Һ
b NH3 ��HCl����H2O
��2��C
��3��6HCl+Fe2O3 == 2FeCl3 + 3H2O
��4����

��ϰ��ϵ�д�
�����Ŀ
 29��С�������ͼ��ʾװ�ý���Ȥζʵ�飮
29��С�������ͼ��ʾװ�ý���Ȥζʵ�飮