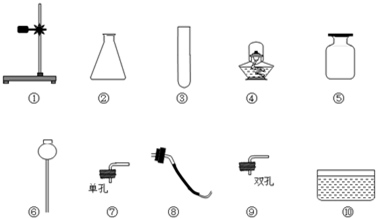
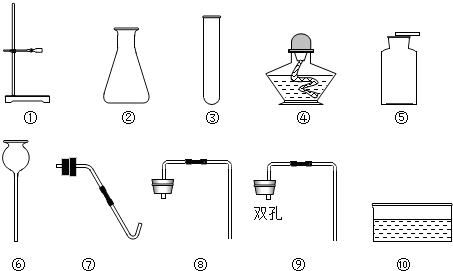
��Ŀ����
�ڳ��л�ѧѧϰ������������������������������̼����ȡ���ռ�������ijУ��ѧ��ȤС���ͬѧ�����淶�IJ���Ҫ�����ú�����װ����ʵ������ȡ����Щ���壮��ͼ������ʵ�����ù�����������ش��������⣺

��1��С��ͬѧ����ȡ����ʱʹ�õ������Ǣ٢ۢܢݢߢ⣬����ȡ��������________���ѧʽ������ȡ������ʱ������Ӧ�Ļ�ѧ����ʽ��________��
��2��С��ͬѧ˳������ȡ���ռ���һƿ������̼����֪���Ѿ�ʹ���������ڢޣ���ô����һ��ʹ������ͼ�����е�________������ţ�����ȡ������̼�Ļ�ѧ����ʽ��________��
�⣺��1����ΪҪ�õ��ƾ��ƣ���������������Ϊ��ȡ������������������̼��������ʱ��ֻ����ȡ����ʱ����Ҫ���ȣ���ȡ�����Ļ�ѧ��Ӧʽ�ǣ�2KClO3 2KCl+3O2����
2KCl+3O2����
�ʴ�Ϊ��O2��2KClO3 2KCl+3O2����
2KCl+3O2����
��2����ȡ������̼�ǹ����Һ��IJ����ȷ�Ӧ���ռ����������ſ����������Ի�Ҫ�õ��ݼ���ƿ�ռ����壬��Ҫ�õ�������ܵ�˫����Ƥ����
�ʴ�Ϊ���ݢCaCO3+2HCl�TCaCl2+CO2��+H2O��
��������1������ʵ��Ҫ�õ��ƾ��ƣ��Ϳ��˽���ȡ����������Ҫ���Ⱥ�õ��ģ����ݴ��жϣ�д����ѧ��Ӧʽ��
��2��������ȡ���ռ�������̼����ķ�Ӧ����������
���������⿼����е��ص�ʵ�飬�����Ͷ�����̼����ȡ���������ͻ�ѧ��Ӧʽ����д������������ʵ���ҩƷ��������������ѧ��Ӧʽ�ȣ�ͬѧ��һ��Ҫ��Ȼ���ģ�
 2KCl+3O2����
2KCl+3O2�����ʴ�Ϊ��O2��2KClO3
 2KCl+3O2����
2KCl+3O2������2����ȡ������̼�ǹ����Һ��IJ����ȷ�Ӧ���ռ����������ſ����������Ի�Ҫ�õ��ݼ���ƿ�ռ����壬��Ҫ�õ�������ܵ�˫����Ƥ����
�ʴ�Ϊ���ݢCaCO3+2HCl�TCaCl2+CO2��+H2O��
��������1������ʵ��Ҫ�õ��ƾ��ƣ��Ϳ��˽���ȡ����������Ҫ���Ⱥ�õ��ģ����ݴ��жϣ�д����ѧ��Ӧʽ��
��2��������ȡ���ռ�������̼����ķ�Ӧ����������
���������⿼����е��ص�ʵ�飬�����Ͷ�����̼����ȡ���������ͻ�ѧ��Ӧʽ����д������������ʵ���ҩƷ��������������ѧ��Ӧʽ�ȣ�ͬѧ��һ��Ҫ��Ȼ���ģ�

��ϰ��ϵ�д�
�����Ŀ