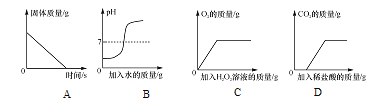
��Ŀ����
����Ŀ����֪A��B��C��D��E�ֱ��dz��л�ѧ���������ֲ�ͬ�������ʣ�����A�ڹ�ҵ�������Ʋ�����B�Ĺ����E��Ũ��Һ������ʵ���ҵĸ������F��һ�ֺ�ɫ���壬����֮�������ͼ��ʾ�Ĺ�ϵ(ͼ����������ʾ���˵������ܷ�Ӧ����������ʾ���ʼ����ת����ϵ���������ʺͷ�Ӧ����δ���)���밴Ҫ��ش��������⣺
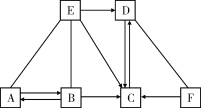
(1)д������D��F�Ļ�ѧʽ��________��__________��
(2)д��A��E�Ļ�ѧ����ʽ��__________________________________��
(3)��B��Һ��E��Һ��ֻ�ϣ�������ҺpH��7����ʱ��Һ�д������ڵ���������_________(�û�ѧ���ű�ʾ)��
���𰸡�H2 CuONa2CO3��H2SO4===Na2SO4��CO2����H2OOH����SO42��
��������
(1)A��B��C��D��E�ֱ��dz��л�ѧ���������ֲ�ͬ�������ʣ�����A�ڹ�ҵ�������Ʋ���������A��̼���ƣ�B�Ĺ����E��Ũ��Һ������ʵ���ҵĸ������A��B�����ת��������B���������ƣ�E�����ᣬ���ᡢ�������ƶ���ת����C������C��ˮ��D��ˮ�����ת��������D��������F��һ�ֺ�ɫ���壬����F������ͭ��������֤���Ƶ���ȷ������D��H2��F��CuO��
(2)A��E�ķ�Ӧ��̼���ƺ����ᷴӦ���������ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��Na2CO3+H2SO4=Na2SO4+H2O+CO2����
(3)��������Һ������������Һ��ֻ�����������ƺ�ˮ��������ҺpH��7����Һ�Լ��ԣ����Դ�ʱ��Һ�д������ڵ���������OH-��SO42����

 ������ϵ�д�
������ϵ�д�����Ŀ����һ���ܱ���������������������̼��ˮ������һ��δ֪����M����һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ��������±���
���� | ���� | ������̼ | ˮ���� | M |
��Ӧǰ����/g | 100 | 1 | 1 | 46 |
��Ӧ������/g | 4 | 89 | 55 | x |
(1)���������غ㶨�ɣ�����Ϊx��ֵӦΪ_____________��
(2)δ֪����Mһ�����е�Ԫ��Ϊ______________________��
(3)��֪δ֪����M����Է�������Ϊ46���Ƴ��仯ѧʽΪ_____________��
(4)�÷�Ӧ�Ļ�ѧ����ʽΪ______________________________��
����Ŀ����1�������ƻ�ѧ���ʷdz����ã������¼�����������Ӧ��
��������⣩����������������Ӧ�IJ�����ʲô��
��֪���Ƶ��������������ƣ�Na2O���������ƣ�Na2O2�����Ƶ������ﶼ����ˮ��Ӧ������ Na2O ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��Na2O��H2O=2NaOH��
Na2O2 ��ˮ��Ӧ�����������ƺ��������仯ѧ����ʽΪ_________��
���������룩����������������Ӧ�IJ������Ϊ��
����һ��______���������Na2O2����������Na2O2 �� Na2O��
��ʵ��̽��һ��
ʵ����� | ʵ������ | ʵ����� |
ȡ���������Ƶ������ ��������������ˮ | ______ | ����һ��ȷ |
����˼��չ����ʵ�������Һ���е���Һ�ʼ��ԣ��������һ��ʵ�鷽��������Һ��
��Ҳ�������������� Na2O2 �� Na2O��Na2O2 ��ϡ���ᷴӦ�Ȼ��ơ�ˮ���������仯ѧ����ʽΪ______��
������̽�������ڿ����з��ýϳ�ʱ�䣬������Ӧ���ɵİ�ɫ������ʲô��
���������룩���ɵİ�ɫ��������ǣ���Na2CO3 ��Na2CO3 �� NaOH
��ʵ��̽����������������Ʒ�������̽������д��ʵ���������ͽ���__________��
��2��С�մ��������Ƹ��ķ��ͼ��������Ȼ�ֽ�����̼���ơ�ˮ�Ͷ�����̼��Ϊ�ⶨijС�մ���Ʒ�Ĵ��ȣ���ȡ 10 g ��Ʒ���ȵ��������ټ���Ϊֹ�����ʲ��μӷ�Ӧ�������ռ���������̼ 2.2 g������Ʒ��С�մ����������__________��