��Ŀ����
��3�֣�ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10����������뵽12.5gˮ���У�����CO2������������ͼ��ʾ��
��������ʾ�� +2HCl=
+2HCl= +H2O��
+H2O��
��1����ͼ�п��Կ�����12.5gˮ�������ᷴӦ�����ɵĶ�����̼����� g��
��2��ˮ����̼��Ƶ����������Ƕ���?
��3������ˮ���г�̼��ƺ�������þ�⣬�������������ʣ��ܽ�12.5 gˮ����������Ҫ��������Ϊ10��������������� (���������һλС��)��
��1��3.3g����2��60%����3��117.7g
���������������1����ͼ�п��Կ�����12.5gˮ�������ᷴӦ�����ɵĶ�����̼�����3.3g��
��2����12.5gˮ����̼��Ƶ�����Ϊx
CaCO3+ 2HCl==CaCl2+H2O+CO2��
100 44
x 3.3g
100��44=x��3.3g
x =7.5g
ˮ����̼��Ƶ���������Ϊ�� ��
��
��3��12.5 gˮ���к���̼���7.5g������������þ12.5-7.5=5g��
�跴Ӧ̼��Ƶ�ϡ���������Ϊm����Ӧ������þ��ϡ���������Ϊn��
CaCO3+ 2HCl==CaCl2+H2O+CO2��
100 73
7.5g m��10%
100��73=7.5g��m��10%
m=54.8g +2HCl=
+2HCl= +H2O
+H2O
58 73
5g n��10%
58��73=5g��n��10%
n=62.9g
���ܽ�12.5 gˮ����������Ҫ��������Ϊ10���������������54.8g+62.9g=117.7g��
��12.5gˮ�������ᷴӦ�����ɵĶ�����̼�����3.3g��ˮ����̼��Ƶ�����������60%���ܽ�12.5 gˮ����������Ҫ��������Ϊ10���������������117.7g��
���㣺���ݻ�ѧ����ʽ���㣻��ѧ��Ӧ�е�������ϵͼ�������ij�ɷֵ�����������
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢�𡣻������ij�ɷֵ���������= ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�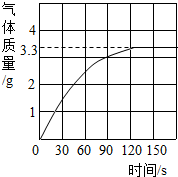 ��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��
��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ�� 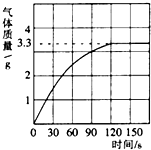 ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��
ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��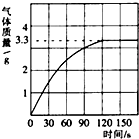 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��