��Ŀ����
2009�����绷���յ�����Ϊ��������Ҫ�㣬�Ž�����Ӧ������仯������ش������й����⣺
��1����ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ������ʯȼ��ȼ��ʹ��ѧ��ת��Ϊ���ܣ��ǵ���������������������Ҫ����Դ�����ǣ�ú��ȼ�ջ����ɶ�������Ͷ����������γ� ��
��2����ʯȼ�ϵ�ȼ�ջ�����������嵼������ЧӦ����Ȼ������Ҫ�ɷ�CH4��Ҳ��һ�ֵ�������ЧӦ�����壬��ȼ�յĻ�ѧ����ʽΪ�������� ��������֪��ͬ��������CH4��CO2��CH4���µ�����ЧӦ�����ԣ�����ͬ�����������������壬��������ЧӦ�����Ե��� ��
��3����Ȼ�����Ķ�����̼�������Ҫ;���Ǣ�ֲ��Ĺ�����á�ֲ���ڹ�����������ն�����̼��ˮ����������(C6H12O6)���������ں�ˮ�����ա����û�ѧ��Ӧ����ʽ��ʾ��������������CO2��;���� �� �� ��
��4���������������ϡ���̼���á���һ������ǣ�����ţ� ��
�ٸ������̭���ܺġ�����Ⱦ��ҵ�� �ڴ�����չ�������磻 �ۿ�������Դ�������ͳ��Դ�� ���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ硣
��1����ʯȼ�ϰ���ú��ʯ�ͺ���Ȼ������ʯȼ��ȼ��ʹ��ѧ��ת��Ϊ���ܣ��ǵ���������������������Ҫ����Դ�����ǣ�ú��ȼ�ջ����ɶ�������Ͷ����������γ� ��
��2����ʯȼ�ϵ�ȼ�ջ�����������嵼������ЧӦ����Ȼ������Ҫ�ɷ�CH4��Ҳ��һ�ֵ�������ЧӦ�����壬��ȼ�յĻ�ѧ����ʽΪ�������� ��������֪��ͬ��������CH4��CO2��CH4���µ�����ЧӦ�����ԣ�����ͬ�����������������壬��������ЧӦ�����Ե��� ��
��3����Ȼ�����Ķ�����̼�������Ҫ;���Ǣ�ֲ��Ĺ�����á�ֲ���ڹ�����������ն�����̼��ˮ����������(C6H12O6)���������ں�ˮ�����ա����û�ѧ��Ӧ����ʽ��ʾ��������������CO2��;���� �� �� ��
��4���������������ϡ���̼���á���һ������ǣ�����ţ� ��
�ٸ������̭���ܺġ�����Ⱦ��ҵ�� �ڴ�����չ�������磻 �ۿ�������Դ�������ͳ��Դ�� ���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ硣
��1������
(2 ) ����ʽ�� CH4
��3������ʽ��
(4 ) ��
(2 ) ����ʽ�� CH4
��3������ʽ��
(4 ) ��
��������1�������������������������壬�������ӵĴ�����ѧ��Ӧ��������ˮ�л��γ����꣮
��2������ȼ�����ɶ�����̼��ˮ��������Ŀ�е���Ϣ����ͬ��������CH4��CO2��CH4���µ�����ЧӦ�����ԡ����Լ����������������Ŀ֮��Ļ����ϵ�ش�
��3����ʯȼ�ϵ�ȼ������Ȼ���еĶ�����̼��������Ҫ��Դ�����Ķ�����̼����Ϣ����д����ʽ��
��4����������ʵ���Ͼ���ȼ�ջ�ʯȼ�ϣ����ɴ���������̼�������ϡ���̼���á���
��𣺽⣺��1��������Ҫ�ɻ�ʯȼ��ȼ�ղ����Ķ���������������������壬�������ӵĴ�����ѧ��Ӧ������ˮ�����ܽ���ɣ�
�ʴ�Ϊ�����ꣻ
��2������ȼ�����ɶ�����̼��ˮ����ѧ��Ӧʽ�ǣ�CH4+2O2 CO2+2H20��
CO2+2H20��
�������Է���������16��������̼����Է�������Ϊ44��������������Է�������֮�Ⱦ��������������ӵ������Ŀ����ͬ�����������������壬�����к��еķ�����Ŀ�ȶ�����̼�࣬��֪��ͬ��������CH4��CO2��CH4���µ�����ЧӦ�����ԣ�����ͬ�����������������壬��������ЧӦ�����Ե��Ǽ��飮
�ʴ�Ϊ��CH4+2O2 CO2+2H20��CH4��
CO2+2H20��CH4��
��3��������������Ϣ������⣬һ���Ƕ�����̼��ˮ���������Ǻ���������һ����ˮ�������̼����̼�ᣮ
�ʴ�Ϊ��6H2O+6CO2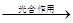 C6H12O6+6O2
C6H12O6+6O2
��H2O+CO2�TH2CO3
��3����������ʵ���Ͼ���ȼ�ջ�ʯȼ�ϣ����Ի�����������Ӷ�����̼�ĺ�������ˣ����Ƿ�������ģ��������������⣮
�ʴ�Ϊ���ڣ�
��2������ȼ�����ɶ�����̼��ˮ��������Ŀ�е���Ϣ����ͬ��������CH4��CO2��CH4���µ�����ЧӦ�����ԡ����Լ����������������Ŀ֮��Ļ����ϵ�ش�
��3����ʯȼ�ϵ�ȼ������Ȼ���еĶ�����̼��������Ҫ��Դ�����Ķ�����̼����Ϣ����д����ʽ��
��4����������ʵ���Ͼ���ȼ�ջ�ʯȼ�ϣ����ɴ���������̼�������ϡ���̼���á���
��𣺽⣺��1��������Ҫ�ɻ�ʯȼ��ȼ�ղ����Ķ���������������������壬�������ӵĴ�����ѧ��Ӧ������ˮ�����ܽ���ɣ�
�ʴ�Ϊ�����ꣻ
��2������ȼ�����ɶ�����̼��ˮ����ѧ��Ӧʽ�ǣ�CH4+2O2
 CO2+2H20��
CO2+2H20���������Է���������16��������̼����Է�������Ϊ44��������������Է�������֮�Ⱦ��������������ӵ������Ŀ����ͬ�����������������壬�����к��еķ�����Ŀ�ȶ�����̼�࣬��֪��ͬ��������CH4��CO2��CH4���µ�����ЧӦ�����ԣ�����ͬ�����������������壬��������ЧӦ�����Ե��Ǽ��飮
�ʴ�Ϊ��CH4+2O2
 CO2+2H20��CH4��
CO2+2H20��CH4����3��������������Ϣ������⣬һ���Ƕ�����̼��ˮ���������Ǻ���������һ����ˮ�������̼����̼�ᣮ
�ʴ�Ϊ��6H2O+6CO2
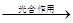 C6H12O6+6O2
C6H12O6+6O2��H2O+CO2�TH2CO3
��3����������ʵ���Ͼ���ȼ�ջ�ʯȼ�ϣ����Ի�����������Ӷ�����̼�ĺ�������ˣ����Ƿ�������ģ��������������⣮
�ʴ�Ϊ���ڣ�

��ϰ��ϵ�д�
�����Ŀ
