��Ŀ����
����Ŀ��ʵ��̨����ҩƷ��KMnO4��ʯ��ʯ��ϡ���ᡢ���ͻ������������(��Ŀ����)����ش��������⣺
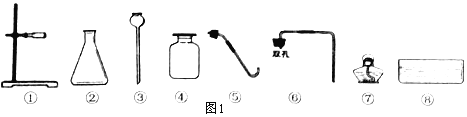
(1)�����ڵ�����___________,�����۵�����____________.
(2)�������������ѡ������ȡ������̼�ķ���װ�ú��ռ�װ��_____(���������)��������̼���ռ�������___________________��
(3)�ռ�һ����ƿ������̼���壬Ѹ��ע��������ʯ��ˮ������ƿ������ƿ�ӣ���������ʯ��ˮ��______��ͬʱ����ƿ�ӱ�÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
(4)������һ����������______����������ȡ�����������ļ��鷽����_______________��
(5)KMnO4�������ʷ����е�______
a.���� b. ������ c. ������ d. ��
���𰸡� ��ƿ ����©�� �ڢۢܢ� �����ſ����� ���� Ca(OH)2+CO2=CaCO3��+ H2O �Թ� �������ǵ�ľ�����뼯��ƿ�У���ľ����ȼ��֤�������� bd
��������(1)�����ڵ���������ƿ�������۵������dz���©����(2)����ȡ������̼�ķ���װ�ú��ռ�װ���Ǣڢۢܢޣ������Һ�巴Ӧ���������ȡ���壬������̼�ܶȱȿ���������̼���ռ������������ſ�������(3)�ռ�һ����ƿ������̼���壬Ѹ��ע��������ʯ��ˮ������ƿ������ƿ�ӣ���������ʯ��ˮ����ǣ�������̼�����ʯ��ˮ��Ӧ������̼��ư�ɫ������ͬʱ����ƿ�ӱ�÷�Ӧ�Ļ�ѧ����ʽΪCa(OH)2+CO2=CaCO3��+ H2O��(4)������һ�������������Թܣ���������ȡ�����������ļ��鷽���ǽ������ǵ�ľ�����뼯��ƿ�У���ľ����ȼ��֤�������������Ǽ����������õķ�����(5)KMnO4�������ʷ����еĻ�������Σ���ѡbd��
�㾦�����ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ��������ȡװ�ã�����������������ܽ��Ժ��ܶ�ѡ��������ռ������������������õķ�����ʹ�����ǵ�ľ����ȼ��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��NH4Cl��Na2SO4���ܽ�ȱ����ܽ���������¡�����˵����ȷ����
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ�� S/g | NH4Cl | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 | 60.2 |
Na2SO4 | 9.6 | 20.2 | 40.8 | 48.4 | 47.5 | 47.0 | |
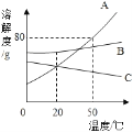
A. ��ΪNH4Cl
B. t3��Ӧ����40����50��
C. �����ʵı�����Һ��t2���µ�t3����Һ������������������
D. �ס��ұ�����Һ��t3���µ�t1����������(�������ᾧˮ)������һ�����
����Ŀ����СӢ�ҵIJֿ���ѷ���һ��������һһ̼�����( NH4HCO3).����һ�����죬СӢ�������ֻ��������еĴ̼�����ζ��ø�Ũ���ˣ���Щ���ʴ���̼����隣����ˣ���鷢�ֱ��ٵĻ��ʰ�װ��û���ܷ⣬����Ҳû�������ڵ��ϣ���û���˽����ֿ��ʹ�á�
Ϊ��̽����Щ���ʼ��ٵ�ԭ��СӢ��ʵ����ȡ��һЩ̼����立�ĩ�������������м��ȣ���һ����۲쵽��ĩ��ȫ��ʧ��ͬʱҲ�ŵ������ִ̼�����ζ.��ĩΪʲô����ʧ��?
(1)��������⣩̼����立�ĩ��ʧ��ԭ����ʲô?
(2)�����룩��̼����立�ĩ�ڲ����Ȼ�����������ɹ�̬���������̬����̼������ڲ����Ȼ���������·����ֽⷴӦ�����ܲ����������а�����һЩ�����
(3)���������ϣ���̼��������ڰ��ʣ����������������ʣ�˵����������__________ (�����)���������ڰ���(��ѧʽNH3)��������Ĵ̼�����ζ����������ˮ����ˮ��Һ�Ǽ��ԣ�������İ�������ʹ����ĺ�ɫʯ���Լ���������NO2Ϊ����ɫ���塣NOΪ��ɫ���壬�ڿ�����������Ӧ��2NO+O2=2NO2
(4)��ʵ���������������ۣ�

ʵ����� | ʵ������ | ʵ����� |
��ȡ����̼��������Թ��м��ȣ���ͼһ��ʾ��������ĺ�ɫʯ����ֽ�ӽ����ܿ� | ����ǿ�ҵĴ̼�����ζ���Թܱ�������ɫҺ������ֽ��������δ������ɫ���� | �ֽ��������______��û��__________ |
�ڰ���ͼ����ʾװ�ü���ʵ�飬ֱ����Ӧ��ȫ | ����ʯ��ˮ����� | �ֽ��������__________ |
(5)��Ӧ�ã������ð����Ļ�ѧ���ʣ���д��ʵ���Ҽ��鰱���ķ���(д��ʵ�������������)��________________________________________��
������������̼����炙��ʣ���Ӧ����α���? ______________________________��
����Ŀ��ij��ѧѧϰС���ͬѧ��һƿ���ڷ����������ƹ���Ļ�ѧ�ɷֲ�������Ȥ�����Ǹ����Լ���ѧ֪ʶ��������̽��:
[�������]��ƿҩƷ�Ļ�ѧ�ɷ���ʲô?
[��������](1)����������������еĶ�����̼��Ӧ�����ʡ�
(2)�������Ƶ��ܽ��������ͼ:
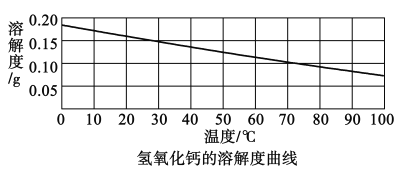
[�������]����һ:Ca(OH)2
�����:__________
������:CaCO3
[ʵ����֤]
ʵ����� | ʵ������ | ʵ����� |
(1)ȡ1g��Ʒ����ʢ��50gˮ���ձ��У���ֽ����,���� | �ձ��ײ��н϶����δ�ܽ� | ��Ʒ�к���CaCO3 |
(2)ȡʵ��(1)���ϲ���Һ�������Թ��У������Թ��еμ�2��3����ɫ��̪��Һ | _____________ | ��Ʒ�к���Ca(OH)2 |
[�ó�����]��ͬѧ��Ϊ�������ȷ��
[��˼����]
(1)��ͬѧ��Ϊ��ͬѧ�Ľ��۲�����,����Ϊ����_____Ҳ������ȷ,ԭ����______��
(2)��ͬѧ��ΪҪ����֤�������ȷ,����������ʵ��:ȡʵ��(1)��δ�ܽ�������Թ��У����Թ��еμ�����ϡ����,����_______,��������ȷ��
[��չ����]Ҫ����֤��Ӧ�����ʵijɷ֣�������֤�����������⣬��Ӧ���Ƿ�Ӧ���Ƿ�________________��