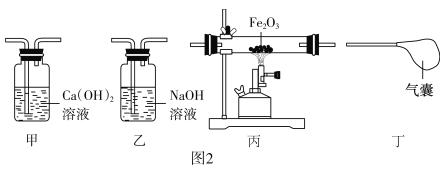
��Ŀ����
����Ŀ�������ó�϶�ʱ���õ����ĺ�ɱ���ɱ�ó档������֪������������ʮɱ���=������Ҳ����˵������������ɱ��������������
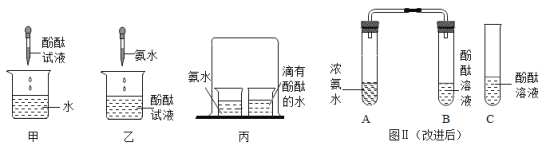
��1��ͨ��ĵ������Ż�����Ϊɱ����������ij�������ǿ�ȼ�����������������_____��
��2���������¼�Ҳ������ɱ��������ijɷ����ж��ֿ�ȼ���ܼ������Ҵ�������д���Ҵ���ȫȼ�յĻ�ѧ����ʽ��_____��
��3�������й�ɱ������������¼�����ȼ�ױ���Ʒ�ڼ����ź�ʹ��ע�������˵���������_____��
a����Ҫ����ڿ�����Դ�ĵط�
b��������ڸ��µĵط�
c��ʹ��ʱ����������Ļ�������ը
���𰸡�ͨ����������� C2H5OH��3O2![]() 2CO2��3H2O c
2CO2��3H2O c
��������
��1��ͨ��ĵ������Ż�����Ϊɱ����������ij�������ǿ�ȼ����������������ǣ�ͨ�������������
��2���Ҵ��ڿ�������ȫȼ������ˮ�Ͷ�����̼�Ļ�ѧ����ʽ��C2H5OH��3O2![]() 2CO2��3H2O
2CO2��3H2O
��3�������й�ɱ������������¼�����ȼ�ױ���Ʒ�ڼ����ź�ʹ��ע�������˵���������
a����Ҫ����ڿ�����Դ�ĵط���������Σ�գ���ѡ����ȷ�������������⣻
b��������ڸ��µĵط���������Σ�գ���ѡ����ȷ�������������⣻
c��ʹ��ʱ����������Ļ�������ȼ����������ը����ѡ������������⣻
��ѡ��c

����Ŀ�����ղ�����������ʵ����̽����
��֪ʶ�عˣ�
��1��д����ʵ���У��ƾ���C2H6O��ȼ������ˮ�Ͷ�����̼�Ļ�ѧ����ʽ��_____��
��2������δȼ�յ�ԭ����_____��
��������⣩����ֽ����������ʵ�飬�ʺϵľƾ���Һ��������������Χ�Ƕ���?
��ʵ�鲽�裩��.����ͼ�������Ʋ�ͬŨ�ȵľƾ���Һ����֪:��ˮ�ƾ����ܶ�Ϊ0.8g/mL��ˮ���ܶ�Ϊ1.0g/mL��
ʵ���� | ��ˮ�ƾ����/mL | ˮ���/mL |
�� | 10 | 5 |
�� | 10 | 10 |
�� | 10 | 15 |
�� | 10 | 20 |
�� | 10 | 30 |
��.�ò�ͬŨ�ȵľƾ���Һ������ֽ��
��.����ͼ1��ʾװ�òⶨ�ƾ�ȼ��ʱ��ֽ���¶ȣ��������ݻ��Ƴ�ͼ2��
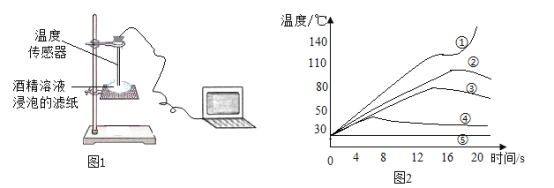
�Իش�:
��3�����ߢ��ڽӽ�20sʱ���¶�ͻȻ��������Ҫԭ����_____
��4��ʵ��۴�Լ��_____��ʱ�ƾ�ȼ��������Ϩ��
��ʵ����ۣ�
��5������ͼ2��Ϣ������ֽ�����ղ�����������ʵ�飬Ϊȷ��ʵ��ɹ������þƾ���Һ��������������������������Χ��_____�����ռ�����������С�����1λ��
����˼��ߣ�
��6������ͼ2�����ߢܺ͢ݣ��������ȼ������������ʶ��_____��
��7����ʵ��ڵľƾ���Һʵ��ʱ������ֽ�������һ�ţ������ֽ���ճɻҽ�������Ҫ��Ũ�Ƚϴ�ľƾ���Һ�ɹ���ɱ�ʵ��Ӧ��_____��