��Ŀ����
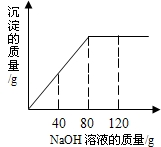 ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ���Ȼ�þ��Һ��Ϊ�˲ⶨ����Һ������������������ȡ��һ�����ĸ���Һ����������μ���������������Ϊ5%������������Һ����Ӧ���������ɳ�������������������������Һ�����Ĺ�ϵ����ͼ��ʾ��
ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ���Ȼ�þ��Һ��Ϊ�˲ⶨ����Һ������������������ȡ��һ�����ĸ���Һ����������μ���������������Ϊ5%������������Һ����Ӧ���������ɳ�������������������������Һ�����Ĺ�ϵ����ͼ��ʾ������Ӧ�Ļ�ѧ����ʽΪMgCl2+2NaOH�T2NaCl+Mg��OH��2����
��1��������������ҺΪ
��2������Ӧ�������Ȼ�þ��Һ������Ϊ100g���������Ȼ�þ��Һ����������������
��������1�����ݷ�Ӧ���������ɳ�������������������������Һ�����Ĺ�ϵͼ���ж�ǡ����ȫ��Ӧ�������۵���������������Һ�������������ݷ�Ӧ�Ļ�ѧ����ʽ�����������������������ɳ���������þ��������
��2���Ȼ�þ��Һ��������������=
��100%���Ȼ�þ�������ɸ��ݻ�ѧ����ʽ����������������ã�
��2���Ȼ�þ��Һ��������������=
| �Ȼ�þ������ |
| ��ȡ�Ȼ�þ��Һ������ |
����⣺��1�����ݷ�Ӧ�����ߣ���֪������80g����������Һʱǡ����ȫ��Ӧ����ʱ��Ӧ�����������Ƶ�����=80g��5%=4g��
�����ɳ���������Ϊx���μӷ�Ӧ���Ȼ�þ������Ϊy
MgCl2+2NaOH�TMg��OH��2��+2NaCl
95 80 58
y 4g x
=
=
x=2.9g��y=4.75g
�ʴ�Ϊ����1��80��2.9
��2�����Ȼ�þ��Һ��������������=
��100%=
��100%=4.75%
���Ȼ�þ��Һ��������������Ϊ4.75%��
�����ɳ���������Ϊx���μӷ�Ӧ���Ȼ�þ������Ϊy
MgCl2+2NaOH�TMg��OH��2��+2NaCl
95 80 58
y 4g x
| 95 |
| y |
| 80 |
| 4g |
| 58 |
| x |
x=2.9g��y=4.75g
�ʴ�Ϊ����1��80��2.9
��2�����Ȼ�þ��Һ��������������=
| �Ȼ�þ������ |
| ��ȡ�Ȼ�þ��Һ������ |
| 4.75g |
| 100g |
���Ȼ�þ��Һ��������������Ϊ4.75%��
������������Ҫ����ѧ�����û�ѧ�����ۺϷ����ͽ��ʵ��������������������Ӧ�ļ�����ʱҪ���淶�ĸ�ʽ�Ͳ�����У���ע���������ȷ�ԣ�

��ϰ��ϵ�д�
�����Ŀ
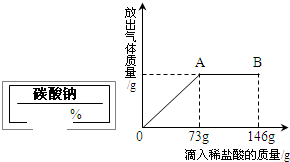 ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��100g����Һ���������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��
ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��100g����Һ���������μ�������������Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��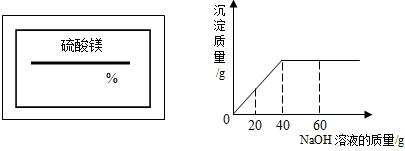
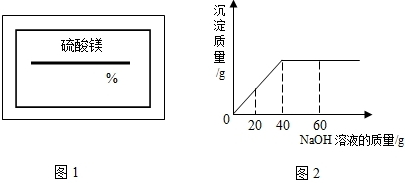
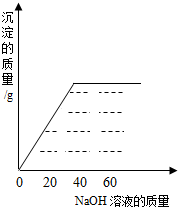 ��2009?�Ͽ���һģ��ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������þ��Һ��Ϊ�˲ⶨ����Һ������������������ȡ��25g����Һ����������μ���������������Ϊ10%��NaOH��Һ����Ӧ���������ɳ���������������NaOH��Һ�����Ĺ�ϵ��ͼ��ʾ������
��2009?�Ͽ���һģ��ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ������þ��Һ��Ϊ�˲ⶨ����Һ������������������ȡ��25g����Һ����������μ���������������Ϊ10%��NaOH��Һ����Ӧ���������ɳ���������������NaOH��Һ�����Ĺ�ϵ��ͼ��ʾ������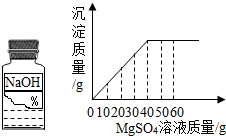 ��2008?��ǽ�����ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ����ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��20g����Һ����������μ���������������Ϊ15%��MgSO4��Һ����Ӧ���������ɳ���������������MgSO4��Һ�����Ĺ�ϵ��ͼ��ʾ����������Һ����������������
��2008?��ǽ�����ijͬѧ��ʵ���ҷ���һƿ��ǩ��ȱ����ͼ��ʾ������Һ��Ϊ�˲ⶨ����Һ������������������ȡ��20g����Һ����������μ���������������Ϊ15%��MgSO4��Һ����Ӧ���������ɳ���������������MgSO4��Һ�����Ĺ�ϵ��ͼ��ʾ����������Һ����������������