��Ŀ����
����Ŀ���������ͼa��b��c���ֹ������ʵ��ܽ�����ߣ��ش���������:
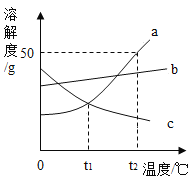
(1)��_______��ʱ��a��c�������ʵ��ܽ����ȡ�
(2)��t2��ʱ����ʢ��50ga���ʵ��ձ��м���50gˮ������ܽ��������Һ������Ϊ______g��
(3)��a�����л�������b���ʣ���ò���___�ķ����ᴿ��(�������½ᾧ�����������ᾧ��)��
(4)t2��ʱ��a��b��c�������ʵı�����Һ��100g�������ܼ��������ɴ�С��˳����_____��
(5)����˵����ȷ����______��
A.��a���ʵı�����Һ��Ϊ��������Һ�������ʵ���������һ����С
B.��t1��ʱc���ʵı�����Һ���µ�t2�棬����Һ������һ����С
C.����һ����������������b������Һ������ȡˮʱ���Ӷ�����������������ȷ�������Ƶ�b��Һ�����ʵ�����������ƫ��
���𰸡� t1 75 ���½ᾧ c>b>a��cba BC
�������������ܽ�����߷��������(1)���ܽ�����߿�֪����t1��ʱ��a��c���������н�������t1��ʱa��c�������ʵ��ܽ�������(2) ���ܽ�����߿�֪��t2��ʱa���ʵ��ܽ��Ϊ50g����100gˮ������ܽ�50ga���ʣ���ô50gˮ������ܽ�25ga���ʣ�����t2��ʱ����ʢ��50ga���ʵ��ձ��м���50gˮ������ܽ��������Һ������Ϊ75g��(3)���ܽ�����߿�֪��a��b���ܽ�ȶ����¶ȵ����߶�����a���¶ȵ�Ӱ��仯�ϴ�b���¶ȵ�Ӱ��仯��С������a�����л�������b���ʣ���ò��ý��½ᾧ�ķ����ᴿ��(4) ���ܽ�����߿�֪��t2��ʱa��b��c�������ʵ��ܽ�ȴ�С��ϵ��a��b��c����t2��ʱ��a��b��c�������ʵı�����Һ��100g�������ܼ��������ɴ�С��˳����c>b>a����cba����(5)A����a���ʵı�����Һ��Ϊ��������Һ�������ʵ�����������һ����С���������¶ȣ���ʱ���������������䣬������B�����ܽ�����߿�֪��C���ܽ�����¶ȵ����߶����ʽ�t1��ʱc���ʵı�����Һ���µ�t2�棬��C��������������Һ������һ����С����ȷ��C�����Ӷ���������ƫ����������ˮ�����ʵ��ƫС�������Ƶ���Һ�����ʵ�����������ƫ������ȷ����ѡBC��

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�