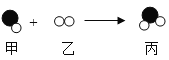��Ŀ����
����Ŀ������ͭ���������������г��õĽ���:CaCO3+2HCl =CaCl2+H2O+CO2��
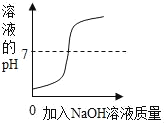
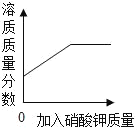
��1�������Ƴ���������Ϊ���������õ�______��;���Ƴ���������Ϊ���������õ�_______��;
��2������ͭ�����Ļ����ǿ�������е�˳����_________����Ԫ�ط��ţ�;
��3����д��һ��ϡ����������������Ӧ�Ļ�ѧ����ʽ:____����Ӧǰ�����Ԫ�صĻ��ϼ�___����������������������������������;
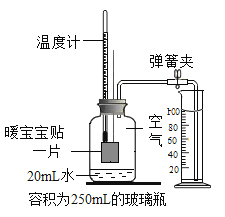
��4�������������ߵĽ�����������ԭ��������_______����������ʯ�л�ԭ������
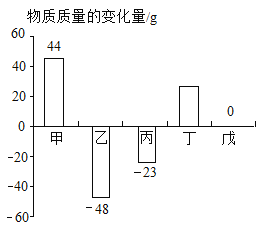
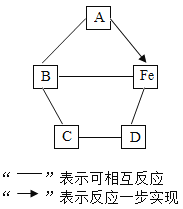
���𰸡���չ�� ������ A1 Fe Cu ����A1>Fe>Cu �� ![]() ��
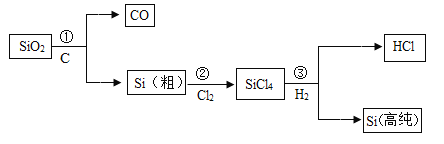
��![]() �� ���� CO ����һ����̼��
�� ���� CO ����һ����̼��
��������
��1�����������õ���չ�ԣ����Կ��Ƴ����������������õĵ����ԣ����Ƴ�������
��2���ɽ����Ļ��˳���֪������ͭ�����Ļ����ǿ�������е�˳����Al��Fe��Cu��
��3���������ᷴӦ�����Ȼ���������������Ԫ����0������Ϊ+2�ۣ��������ᷴӦ�����Ȼ�������������Ԫ����0������Ϊ+3�ۣ���Ӧ�Ļ�ѧ����ʽ��Fe+2HCl=FeCl2+H2����
��4���ڸ�¯��������һ����̼����������ԭΪ����

����Ŀ����ѧ��ѧ������ѧ���úû�ѧ��ѧϰС��Ϊ̽����������ͭ�Ļ��ǿ������չ�����»��
���������ϣ��������ڳ�������������е�������Ӧ�������ܵ���������Ĥ��
���Ա�ʵ�飩
��� | ���� | ���� |
�� | ������δ��ĥ����˿����CuSO4��Һ�� | ���������� |
�� | �������ĥ�����˿����CuSO4��Һ�� | ��˿����������ɫ���� |
�� | ������δ��ĥ����˿����CuCl2��Һ�� | ��˿����������ɫ���� |
��1���������ڳ�������������е�������Ӧ�������ܵ���������Ĥ����д����ط�Ӧ����ʽ_____�Ƚ�ʵ���Һ�ʵ��_____����ס��������ɵ�֪����ĥ���ƻ���������Ĥ��
��2��ʵ�����з�Ӧ�Ļ�ѧ����ʽΪ_____���ݴ˿�֪�������Al��Cu_____���ǿ������������
��3��С��ͬѧ��ʵ�����������з�������ΪH2O����������Ĥ���ƻ����á����˹۵����ϱ�����ͬѧ����������_____��
���²���̽����
С��ͬѧ���ʵ������������ۺ�²⣺Cl���ƻ�����������Ĥ��
Ϊ����˲²��Ƿ���ȷ��������������֧�Թ��м�����ͬ��CuSO4��Һ�������������δ��ĥ����˿��Ȼ��������µ�̽����
���� | ���� | ���� | ���� |
��1����һ֧�Թ����ټ���NaCl���� | ��˿����������ɫ���� | ��������Ĥ���ƻ� | Na+��_____���ƻ���������Ĥ |
��2������һ֧�Թ����ټ���Na2SO4���� | _____ | ��������Ĥδ���ƻ� | Na+��SO42���������ƻ���������Ĥ |
�������뷴˼��
�ó����ۣ�ǰ���²�_____�����ȷ������ȷ������
�ܽᷴ˼������̽����������˱ȽϷ��Ϳ��Ʊ�������