��Ŀ����
ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����
[��������]�����������ʷ�����Ӧ�Ļ�ѧ����ʽΪ___________���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᡣ
[ʵ��̽��]��ȷ����Һ���Ƿ�������
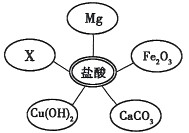
��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ��������ͼ��ʾ���������ʣ���������x�����ָʾ���е� ��Һ��
[��������]�����������ʷ�����Ӧ�Ļ�ѧ����ʽΪ___________���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᡣ
[ʵ��̽��]��ȷ����Һ���Ƿ�������
��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ��������ͼ��ʾ���������ʣ���������x�����ָʾ���е� ��Һ��
��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽ȷ����Һ��һ��û�����ᡣ
��ȷ����Һ���Ƿ���Na2CO3
ijͬѧѡ��_________�����Һ��pH=10��ȷ����Һ��һ������Na2CO3��
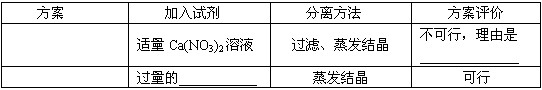
�紦����Һ�������������ӷ�Һ�еõ�������NaCl�����������ʵ�鷽����ơ�
��ȷ����Һ���Ƿ���Na2CO3
ijͬѧѡ��_________�����Һ��pH=10��ȷ����Һ��һ������Na2CO3��
�紦����Һ�������������ӷ�Һ�еõ�������NaCl�����������ʵ�鷽����ơ�
[��������]Na2CO3+2HCl==2NaCl+H2O+CO2��
[ʵ��̽��](һ)��ʯ��
�������ݲ���(����������ޱ仯)
(��)pH��ֽ(��pH��)
[ʵ��̽��](һ)��ʯ��
�������ݲ���(����������ޱ仯)
(��)pH��ֽ(��pH��)


��ϰ��ϵ�д�
�����Ŀ
ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����
��������
�����������ʷ�����Ӧ�Ļ�ѧ����ʽΪ______���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3��HCl
ʵ��̽��
��һ��ȷ����Һ���Ƿ�������
��1��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ�ǿ�ѡ��ʯ�Mg��______��______��______����ͬ���Ļ������
��2��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽��Һ��______��ȷ��һ��û�����ᣮ
������ȷ����Һ���Ƿ���Na2CO3
ijͬѧѡ��______�����Һ��pH=l0��ȷ����Һ��һ������Na2CO3��
������������Һ����������
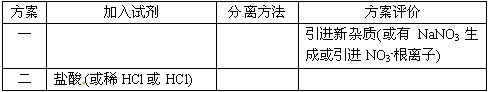
���ӷ�Һ�еõ�������NaCl������ɶ�����ʵ�鷽����Ƶ����ۣ�
| ���� | �����Լ� | ���뷽�� | �������� ���л��У���˵�����ɣ� |
| һ | ����Ca��NO3��2��Һ | ���ˡ������ᾧ | ______ |
| �� | ������HCl��Һ | �����ᾧ | ______ |
 ��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����
��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����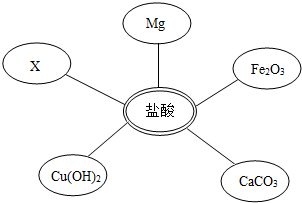 ͬѧ����Na2CO3��Һ��Ũ�����о�����������ķ�Ӧԭ��ʱ���Է�Һ�ɷֽ���̽����
ͬѧ����Na2CO3��Һ��Ũ�����о�����������ķ�Ӧԭ��ʱ���Է�Һ�ɷֽ���̽����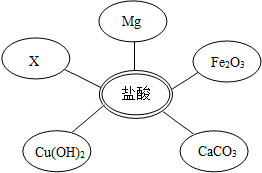 ��2012?���У�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ�����̽����
��2012?���У�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ�����̽����