��Ŀ����
��һ�����ı�����C3H8O������������һ���ܱյ���������ȼ����ַ�Ӧ����÷�Ӧǰ������ʵ��������±���| ���� | ���� | ���� | ˮ | ������̼ | X |
| ��Ӧǰ����/g | 6.0 | 12.8 | |||
| ��Ӧ������/g | 7.2 | 8.8 | a |
A������a��ֵһ����2.8
B��������X��һ��������Ԫ��
C����2.0g������ȫ��Ӧ������2.4gˮ
D����6.0g������14.4g������ַ�Ӧ������X����
���𰸡����������ݱ�������ɿ�֪������ȫȼ�����ɶ�����̼��ˮ���ٸ��ݱ����е����ݼ������غ��֪����Ӧ�б�����������Ӧ���ɶ�����̼��ˮ��X�������ʣ���X������Ϊ12.8g+6.0g-7.2g-8.8g=2.8g��ͬʱҲ�����������غ��е�Ԫ���غ��ж�X��Ԫ�������������ݱ�����ȼ�շ���ʽ��2C3H7OH+9O2  6CO2+8H2O�ɶ���ص�������ϵ���м��㣮
6CO2+8H2O�ɶ���ص�������ϵ���м��㣮
����⣺A���������غ㶨�ɿ�֪���μӷ�Ӧ�ı���������������������֮�͵��ڷ�Ӧ���ɵĶ�����̼��ˮ��X������֮�ͣ���12.8g+6.0g-7.2g-8.8g=2.8g����a=2.8����A˵����ȷ��
B�����������Ԫ�ص�����Ϊ6.0g× ×100%=0.8g��ˮ����Ԫ�ص�����Ϊ7.2g×
×100%=0.8g��ˮ����Ԫ�ص�����Ϊ7.2g×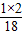 ×100%=0.8g������Ԫ�������غ��֪����X��û����ԭ�ӣ���B˵������
×100%=0.8g������Ԫ�������غ��֪����X��û����ԭ�ӣ���B˵������
C��������е����ݿ�֪��6.0g����g������7.2gˮ������2.0g����ȼ��һ��������2.4gˮ����C˵����ȷ��
D����6.0�˱�����C3H8O����ȫȼ����Ҫ����������Ϊx��
2C3H7OH+9O2 6CO2+8H2O
6CO2+8H2O
2×60 9×32
6.0g x
 =
=
���x=14.4�ˣ����û��X���ɣ���D˵����ȷ��
��ѡB��
���������⿼��ѧ�����ñ��������ʵ������������غ��������������ȷ��ѧ��Ӧ����ʽ������ǡ����ȫ��Ӧ�������ȼ�Ԫ�������ļ����ǽ��Ĺؼ���
 6CO2+8H2O�ɶ���ص�������ϵ���м��㣮
6CO2+8H2O�ɶ���ص�������ϵ���м��㣮����⣺A���������غ㶨�ɿ�֪���μӷ�Ӧ�ı���������������������֮�͵��ڷ�Ӧ���ɵĶ�����̼��ˮ��X������֮�ͣ���12.8g+6.0g-7.2g-8.8g=2.8g����a=2.8����A˵����ȷ��
B�����������Ԫ�ص�����Ϊ6.0g×
 ×100%=0.8g��ˮ����Ԫ�ص�����Ϊ7.2g×
×100%=0.8g��ˮ����Ԫ�ص�����Ϊ7.2g×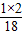 ×100%=0.8g������Ԫ�������غ��֪����X��û����ԭ�ӣ���B˵������
×100%=0.8g������Ԫ�������غ��֪����X��û����ԭ�ӣ���B˵������C��������е����ݿ�֪��6.0g����g������7.2gˮ������2.0g����ȼ��һ��������2.4gˮ����C˵����ȷ��
D����6.0�˱�����C3H8O����ȫȼ����Ҫ����������Ϊx��
2C3H7OH+9O2
 6CO2+8H2O
6CO2+8H2O2×60 9×32
6.0g x
 =
=
���x=14.4�ˣ����û��X���ɣ���D˵����ȷ��
��ѡB��
���������⿼��ѧ�����ñ��������ʵ������������غ��������������ȷ��ѧ��Ӧ����ʽ������ǡ����ȫ��Ӧ�������ȼ�Ԫ�������ļ����ǽ��Ĺؼ���

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
��2011?��������һ�����ı�����C3H8O������������һ����յ���������ȼ����÷�Ӧǰ������ʵ��������±���
|
��һ�����ı�����C3H8O������������һ���ܱյ���������ȼ����ַ�Ӧ����÷�Ӧǰ������ʵ����������
|
��һ�����ı�����C3H8O������������һ����յ���������ȼ�����ɶ�����̼��ˮ������һ��δ֪����W���ֲ�÷�Ӧǰ������ʵ��������£�
|