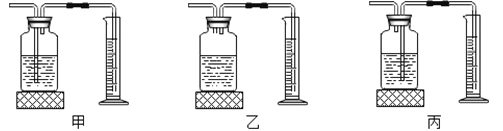
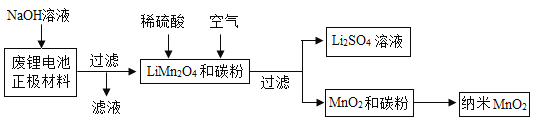
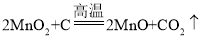��Ŀ����
����Ŀ������۱�ʶ����̽�����ǻ�ѧѧ�Ƶĺ�������֮һ���ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ���Ҫ˼ά��ʽ��
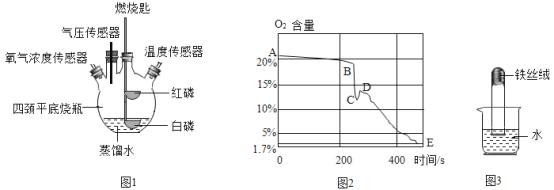
��1�������������ӽṹʾ��ͼ���ش�����
��A��B��C��E�����������ӵ���________�������ӷ��ţ���
����D��ʾijԪ�ص�ԭ�ӣ���������ڻ�ѧ�仯������________��ѡ��õ�����ʧȥ�������ӡ�
����DΪ���ӣ���BD���ɵĻ�����Ļ�ѧʽΪ________��
��2����������������Һ��ϡ���ᷴӦ����ʾ��ͼ��д������������Һ��ϡ���ᷴӦ�Ļ�ѧ����ʽ________��

���𰸡�S2�� �õ� NaCl 2NaOH+H2SO4=Na2SO4+2H2O
��������
��1����A��B��C��E��
A��������=���������=1������ԭ�ӣ�
B��������=11>���������=10���������ӣ����������ӣ�
C��������=���������=16������ԭ�ӣ�
E��������=16<���������=18���������ӣ����������ӣ�
�����������ӵ��������ӣ�����Ϊ![]() ��
��
����D��ʾijԪ�ص�ԭ�ӣ���m=17-2-8=7>4���������ڻ�ѧ�仯�����õ����ӡ�
����DΪ���ӣ�������=17����m=8����DΪ�����ӣ���BΪ�����ӣ���BD���ɵĻ�����Ϊ�Ȼ��ƣ���ѧʽΪ![]() ��
��
��2���۲�����������Һ��ϡ���ᷴӦ����ʾ��ͼ�����������غ㶨�ɿ�֪����Ϊ�����ӣ���Ϊ��������ӣ�������������Һ��ϡ���ᷴӦ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�