��Ŀ����
����Ŀ��2008��5��12�գ��Ĵ��봨����������8.0������ȫ����������֣���־�ɳǡ�Ϊȷ�������֮�����ߣ��������������ȼ���Ư�۵ȶ�����ˮ�Ƚ���������
��1��Ư�۵���Ч�ɷ���Ca��ClO��2 �� ��Ԫ�صĻ��ϼ����������Ѹ�+2�ۣ���-2�ۣ�
��2��ClO2 ����һ������ˮ�����������ҹ��ɹ����Ƴ���ȡClO2 ���·������䷴Ӧ���۹���ͼ���£�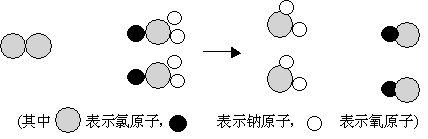
�Իش�
ClO2 ����Ԫ�صĻ��ϼ�Ϊ��
���ݷ�Ӧ���۹���ͼд����Ӧ�Ļ�ѧ����ʽ��
���𰸡�
��1����1
��2����4,Cl2+2NaClO2=2ClO2+2NaCl
����������1�����������ܼ۴�����Ϊ��ԭ��Ư�۵���Ч�ɷ���Ca��ClO��2����Ԫ�صĻ��ϼ��ǩv��2�w��[x���v��2�w]��2��0, x����1��
��2��ClO2 ����һ������ˮ�����������ҹ��ɹ����Ƴ���ȡClO2 ���·���, ClO2 ����Ԫ�صĻ��ϼ�Ϊ x������2����2��0, x����4�����ݷ�Ӧ���۹���ͼд����Ӧ�Ļ�ѧ����ʽ��Cl2+2NaClO2=2ClO2+2NaCl.
�ʴ�Ϊ����1����1����2����4��Cl2+2NaClO2=2ClO2+2NaCl.
�����ڻ��������������ϼ۴�����Ϊ�����ָ��Ԫ�ػ��ϼ۽��з�������⣮

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�