��Ŀ����
����Ŀ��Ϊ�˲ⶨ����ʯ��Ʒ�иƵ���������(���ʲ�����Ԫ�أ�������ˮ��Ҳ�����������ʷ�����Ӧ)������������ʵ�顣��ش��������⡣
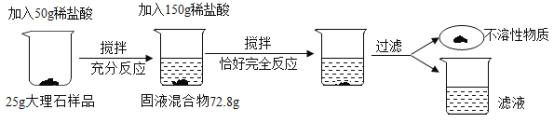
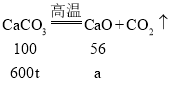
��1������ʵ������з�����Ӧ�Ļ�ѧ����ʽΪ________��
��2��������֪�����г�����һ�����ӵ�ϡ���������ʵ�����(x)�ı���ʽ________��
��3���˴���ʯ��Ʒ�и�Ԫ�ص���������________��
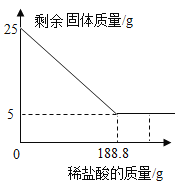
��4������Ӧ�����Һ�м�10.8gˮ��������Һ�����ʵ���������Ϊ________��
��5��������������ʯ��Ʒ750t�������Ƶú����ʵ���ʯ�ҵ�����Ϊ________��
���𰸡�CaCO3+2HCl=CaCl2+H2O+CO2�� ![]() 32% 10% 486t
32% 10% 486t
��������
̼��������ᷴӦ�����Ȼ��ơ�������̼��ˮ��̼��Ƹ������������ƺͶ�����̼��
��1������ʵ������з�����Ӧ��̼��������ᷴӦ�����Ȼ��ơ�������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+H2O+CO2����
��2���������غ㶨�ɿ�֪����Ӧ�������������������������������ٵ�Ϊ���ɵ��������������һ�����ӵ�ϡ��������ɶ�����̼������Ϊ![]()
��μӷ�Ӧ���������Ϊx
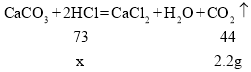
![]()
![]()
�ʵ�һ�����ӵ�ϡ���������ʵ�����(x)�ı���ʽ![]() ��
��
��3����һ�μ���50g���������2.2g������̼����������150g�����ǡ����ȫ��Ӧ��˵�������ɶ�����̼������Ϊ![]() ��25g����ʯ��ȫ��Ӧ�����ɶ�����̼������Ϊ
��25g����ʯ��ȫ��Ӧ�����ɶ�����̼������Ϊ![]()
��μӷ�Ӧ̼��Ƶ�����Ϊx1�������Ȼ��Ƶ�����Ϊy
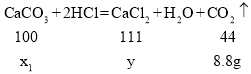
![]()
![]()
![]()
![]()
̼����и�Ԫ�ص���������Ϊ![]()
�ʴ˴���ʯ��Ʒ�и�Ԫ�ص���������Ϊ![]()
��4������Ӧ�����Һ�м�10.8gˮ����������Һ�����ʵ���������Ϊ![]()
��5������ʯ��Ʒ��̼��Ƶ���������Ϊ![]()
����ʯ��Ʒ��̼��Ƶ�����Ϊ![]()
��������������ʯ��Ʒ750t�������Ƶ������Ƶ�����Ϊa
![]()
![]()
�����Ƶú����ʵ���ʯ�ҵ�����Ϊ![]()

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�����Ŀ��ijУ��ȤС��ͬѧ�����г����ᡢ��ε�����̽��ʵ��ʱ������ʵ��̨�ϰڷŵ�ҩƷ�У���һƿװ��Һ���Լ�ƿδ��ƿ���ұ�ǩ������ͼ��ʾ��������С��ͬѧ��������ƿ��Һ����ʵ��̽����
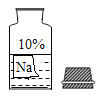
��������⣩���Լ��ijɷ���ʲô��
���������ۣ����������ǩ������жϣ���ƿ�Լ���������______������ĸ��
A �� B �� C ��
���������ϣ�
I�����л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2CO3��NaHCO3
����Na2CO3��NaHCO3��Һ���ʼ��ԡ�
�������£�20����ʱ�������������ʵ��ܽ�ȵ��������£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�ܽ��/g | 36 | 109 | 215 | 9.6 |
���ó����ۣ�С�������Լ�ƿ��ע��������������10%���ϱ����ܽ�ȵ������ж���ƿ�Լ���������______
���������룩��������NaOH��Һ����������Na2CO3��Һ����������NaCl��Һ
����Ʋ�����ʵ�飩
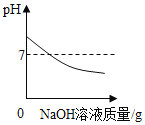
��1��Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����pH��7�������______����
��2��СǿΪ��ȷ������Һ�ijɷ֣����ֽ���������ʵ�飺
�������� | ʵ������ | ���ۼ���ѧ����ʽ |
ȡ�����Թ��У��μ�������___________�����Լ������ƣ� | �������������� | ���� ����ȷ |
��ʦָ���ý��۲��Ͻ�������������������Һ�ڿ����г��ڷ��ûᷢ�����ʣ����ʺ�Ҳ�ܲ�������������д�����������ڿ����б��ʵĻ�ѧ����ʽ��______��
������̽������ȡ�����������CaCl2��Һ���۲쵽��______�����������һ�����Ŀ����_____�����ú�ȡ�ϲ���Һ��������ɫ��̪��Һ����Һ�ʺ�ɫ��
��ʵ����ۣ���ƿ��Һԭ����______��
��̽����ʾ����ʵ��ʱȡ��ҩƷ��Ӧ______��
����Ŀ��20��Cʱ��ij�����ܽ���ˮ��ʵ���������±���������������ȷ���ǣ� ��
ʵ����� | ˮ������/g | ��������ʵ�����/g | ʣ��δ�ܽ���������/g |
�� | 10 | 2 | 0 |
�� | 10 | 3 | 0 |
�� | 10 | 4 | 0.4 |
�� | 10 | 5 | 1.4 |
A.��Һ�����ɴ�С��˳��Ϊ�ܣ��ۣ��ڣ���
B.20��ʱ10g������Һ����3.6g������
C.20��ʱ10gˮ����ܽ�3.6g������
D.��������Һ��������������Ϊ20%