��Ŀ����
ͭ�������������õĽ���֮һ��
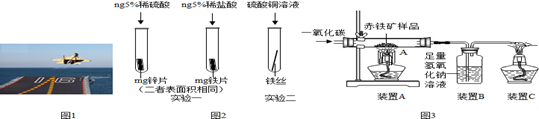
��1������ͭ��Ʒ�У����ý��������Ե����� ��������ĸ��ţ���

A��ͭ�ʽ��� B��ͭ���� C��ͭ���
��2����ʪ����ͭ����ԭ��������ͭ��Һ������Ӧ���÷�Ӧ����������Ӧ����Ϊ�� ����
��3�����÷Ͼɵ��ͭñ���� Cu��Zn����ȡ����ͭ��Cu�������õ�����п��Һ����Ҫ�������£���Ӧ��������ȥ����
��֪��2Cu+2H2SO4+O2 2CuSO4+2H2O
2CuSO4+2H2O
�ٹ��̢��з���������������� ����
�ڹ��̢���������������÷�Ӧ�Ļ�ѧ����ʽΪ ���� ��
��A~D�к�ͭ��п����Ԫ�ص��������� ��������ĸ��ţ���
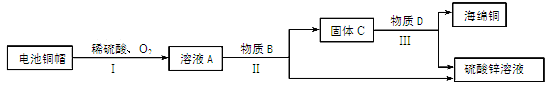
��1������ͭ��Ʒ�У����ý��������Ե����� ��������ĸ��ţ���

A��ͭ�ʽ��� B��ͭ���� C��ͭ���
��2����ʪ����ͭ����ԭ��������ͭ��Һ������Ӧ���÷�Ӧ����������Ӧ����Ϊ�� ����
��3�����÷Ͼɵ��ͭñ���� Cu��Zn����ȡ����ͭ��Cu�������õ�����п��Һ����Ҫ�������£���Ӧ��������ȥ����

��֪��2Cu+2H2SO4+O2
 2CuSO4+2H2O
2CuSO4+2H2O �ٹ��̢��з���������������� ����
�ڹ��̢���������������÷�Ӧ�Ļ�ѧ����ʽΪ ���� ��
��A~D�к�ͭ��п����Ԫ�ص��������� ��������ĸ��ţ���
��1��C ��2���û���Ӧ ��3���ٹ��� ��Zn+H2SO4= ZnSO4+H2�� ��AC
�����������1������ͭ��Ʒ�У����ý��������Ե���Cͭ�������2����ʪ����ͭ����ԭ��������ͭ��Һ������Ӧ�����ݷ�ӳ���ص㣬֪�÷�Ӧ����������Ӧ����Ϊ�û���Ӧ����3������Ϊ���̢��������ֹ���C�����Բ���������Ϊ���ˣ��ڹ��̢���������������÷�Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4= ZnSO4+H2������A~D�к�ͭ��п����Ԫ�ص�������AC��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ