��Ŀ����
����Ŀ���ᡢ��������������������Ź㷺��Ӧ�á�
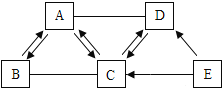
��1�����й����ᡢ��ε�˵����ȷ����______��
A �����֮�䶼�ܷ������ֽⷴӦ B �ܹ������κ�ˮ�ķ�Ӧ�������кͷ�Ӧ
C ��ͼ������Ԫ�� D �ζ����ɽ������Ӻ�������ӹ��ɵ�
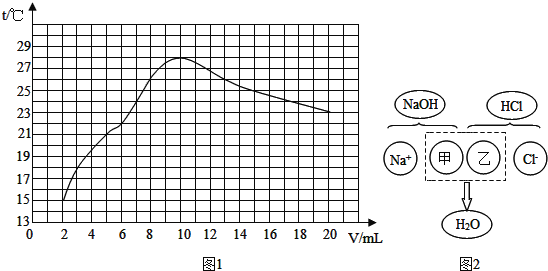
��2����ˮ���������ᾧ�ƵõĴ����л������Ȼ�þ���Ȼ��Ƶ����ʣ�������з�����ᴿ��������ڹ�ҵ���������ǵ��ճ���������ᴿ�IJ���������ͼ��
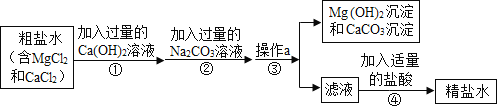
�ٺ�ˮ����ѡ�������ᾧ�������ý��½ᾧ����������______��
��д������ͼ�м������Ca(OH)2��Һ�������Ļ�ѧ��Ӧ����ʽ______��������м������������Ŀ����ʲô��______
�۲���a���õ��IJ�����������ʲô��______
��3�����ᴿ��NaCl����200����������Ϊ0.9%��������ˮ����Ҫ�Ȼ��Ƶ�����Ϊ______�ˡ���������Һ�Ĺ����У�����Ͳ��ȡˮʱ���Ӷ���������������ȷ������������Һ����������______0.9%��������������С������������������
��4��Ϊ�ⶨ���ֱ��ʵ��ռ���Ʒ���������Ƶ�������������ȡ�ù�����Ʒ10g������50gϡ�����У�ǡ����ȫ��Ӧ����Ӧ����Һ������Ϊ57.8g���������Ʒ���������Ƶ���������Ϊ______��
���𰸡�C �Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻�� MgCl2+Ca(OH)2�TMg(OH)2��+CaCl2 ��ȥ������̼���ƺͷ�Ӧ���ɵ��������� �ձ���©���������� 1.8 С�� 47%
��������
��1����������εĶ�������ʽ��з�����
��2�����ݴ����ᴿ�Ĺ��̽��з������
��3����������һ��������������Һ�IJ������з�����
��4�����ݻ�ѧ����ʽ�ļ�����н��
��1��A�������֮�䷢�����ֽⷴӦ���������ģ����������������塢ˮ������������һ�֣���ѡ��˵������ȷ��
B����ͼӦ�����κ�ˮ�ķ�Ӧ���кͷ�Ӧ����ѡ˵������ȷ��
C����ͼ������Ԫ�أ���ѡ��˵����ȷ��
D���β������ɽ������Ӻ�������ӹ��ɵģ������Ȼ�����Σ����в����н������ӣ���ѡ��˵������ȷ��
��ѡ��C��
��2���ٺ�ˮ����ѡ�������ᾧ�������ý��½ᾧ�����������Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻����Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻��
������ͼ�м������Ca(OH)2��Һ�У��������ƺ��Ȼ�þ��Ӧ����������þ�������Ȼ��ƣ������Ļ�ѧ��Ӧ����ʽΪ��MgCl2+Ca(OH)2�TMg(OH)2��+CaCl2�����MgCl2+Ca(OH)2�TMg(OH)2��+CaCl2��
������м������������Ŀ���ǣ���ȥ������̼���ƣ���ȥ̼���ƺ��������Ʒ�Ӧ���ɵ��������ơ������ȥ������̼���ƺͷ�Ӧ���ɵ��������ơ�
�۲���a�ǹ��ˣ��õ��IJ������������ձ���©����������������ձ���©������������
��3�����ᴿ��NaCl����200����������Ϊ0.9%��������ˮ����Ҫ�Ȼ��Ƶ�����Ϊ��200g��0.9%=1.8g����������Һ�Ĺ����У�����Ͳ��ȡˮʱ���Ӷ������ᵼ����ȡˮ�����ƫ����������Һ����������С��0.9%�����1.8��С�ڡ�
��4����̼��������Ϊx��
��Ӧ���ɶ�����̼����Ϊ��10g+50g-57.8g=2.2g��
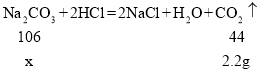
![]() =
=![]()
x=5.3g
����Ʒ���������Ƶ���������Ϊ��![]() ��100%=47%��
��100%=47%��
���47%��

 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�