��Ŀ����
����Ŀ���Ʊ���Ϊ�����еġ��ֽ����������
�������еĸƣ�
��1������ȱ�Ʋ��ᵼ��_____������ĸ����ͬ����
A ٪��֢ B ���Ͳ� C ��������
��2�������и�Ԫ����Ҫ�����ڹ����������У����ǻ������[Ca10��PO4��6��OH��2]��ʽ���ڣ������������PO4�����ϼ�Ϊ_____��
�����ʸƣ�
������⣺�ƵĻ�����ܶ࣬Ϊʲô���Ǻ��������ʸ��أ�
˼�����룺�ƵĻ�ѧ���ʻ��á�
ʵ����֤һ��

�������ϣ���һ����ѧ��Ӧ�У���Ԫ�ػ��ϼ����ߣ�ͬʱ����Ԫ�ػ��ϼ۽��͡�
��3��ʵ����ۣ���д������ˮ��Ӧ�Ļ�ѧ����ʽ_____��
���÷�Ӧ_____������ա��ų�����������
��������еĸƣ�
������⣺һ����ʯ�Ҹ������ʹ��һ��ʱ����������Щ���壿
������裺�����к��������ơ��������ơ�̼��ơ�
ʵ����֤����
��4��ȡ������Ʒ����ˮ�У����¶��������ߣ�����_____���μӷ�̪��Һ���죻����������ϡ���ᣬ��۲쵽_____��������к���̼��ơ�
��5��С����Ϊ���ж��Ƿ����������ƣ�������_____��
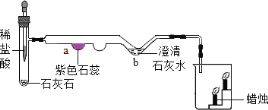
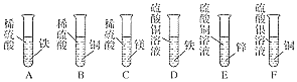
��6��Ϊ�˽�һ��ȷ��������Ʒ�ijɷּ�������ʵ��С��������ͼ��ʾ��װ�ã��г�����ʡ�ԣ�����ʵ�顣

�������ϣ��������Ƽ�����580�桢̼��Ƽ�����825��ֱ�ֽ��������������
��ʵ��ǰ����Ҫ_____����װ��ҩƷ��
��װ��E��������_____��
�����µ������ʹװ��B�з�Ӧ��ȫ����ͨһ��ʱ���������÷�Ӧǰ��װ��C��D�ֱ�����9.0g��11.0g����װ��B�з�Ӧ��ʣ��Ĺ����������������ˮ���ܽ⣬�������ù��壨����ˮ������18.0g����ԭ������Ʒ�и��ɷֵ�����������д��������̣�_____��
���𰸡�A -3 Ca+2H2O=Ca��OH��2+H2�� �ų� ������ �����ݲ��� ��������ˮ��Ӧ���ɵ�����������ҺҲ�ʼ��� ���װ�õ������� ��ֹ�����е�CO2��H2O����D ԭ������Ʒ�к��������Ƶ�����Ϊ37g����̼��Ƶ�����Ϊ25g���������Ƶ�����Ϊ14g��
��������
��1������ȱ�ƻᵼ�����Ͳ��������ɣ����ᵼ��٪��֢����ѡ��A��
��2����Ԫ����+2�ۣ���Ԫ����-2�ۣ���������-1�ۣ���������Ļ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬�ɵã���+2����10+6x+��-1����2=0����x=-3�ۣ����-3��
��3���ٸ���ˮ��Ӧ�Ļ�ѧ����ʽ��Ca+2H2O=Ca��OH��2+H2�������Ca+2H2O=Ca��OH��2+H2����
�ڿ�ʼʱ��Һ����ǣ����º��ɳ�����Һ������Ϊ���������ܽ�������¶����߶���С����˸÷�Ӧ���ڷ��ȷ�Ӧ������ų���
��4��ȡ������Ʒ����ˮ�У����¶��������ߣ����������ƣ��������ɵ�����������Һ�Լ��ԣ���˵μӷ�̪��Һ���죻����������ϡ���ᣬ��۲쵽�����ݲ���������庬��̼��ƣ���������ƣ������ݲ�����
��5����������ˮ��Ӧ���ɵ�����������ҺҲ�ʼ��ԣ�������ж��Ƿ����������ƣ������������ˮ��Ӧ���ɵ�����������ҺҲ�ʼ��ԣ�
��6����ʵ��ǰ����Ҫ���װ�õ������ԣ���װ����Ʒ��������װ�õ������ԣ�
��װ��E��Ϊ�˷�ֹ�����еĶ�����̼��ˮ��������װ��D��Ӱ��ʵ�����������ֹ�����е�CO2��H2O����D��
�ۿ��¼���ʹװ��B�з�Ӧ��ȫ����ͨһ��ʱ���������÷�Ӧǰ��װ��C��D�ֱ�����9.0g��11.0g�����������Ʒֽ�����ˮ������Ϊ9.0g��̼��Ʒֽ����ɶ�����̼������Ϊ11.0g�����������Ƶ�����Ϊx���ֽ����ɵ������Ƶ�����Ϊy��̼��Ƶ�����Ϊz���ֽ����ɵ������Ƶ�����Ϊm����

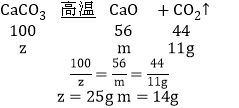
��װ��B�з�Ӧ��ʣ��Ĺ����������������ˮ���ܽ⣬�������ù��壨����ˮ������18g����μӷ�Ӧ�������Ƶ�����Ϊn����
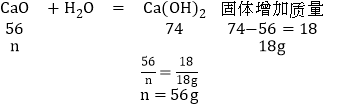
��ԭ������Ʒ��CaO������Ϊ56g-28g-14g=14g
�����Ϸ�����֪��ԭ������Ʒ�к��������Ƶ�����Ϊ37g����̼��Ƶ�����Ϊ25g���������Ƶ�����Ϊ14g��
��ԭ������Ʒ�к��������Ƶ�����Ϊ37g����̼��Ƶ�����Ϊ25g���������Ƶ�����Ϊ14g��