��Ŀ����
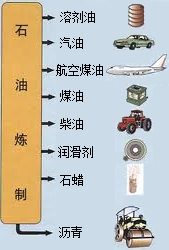 ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�
ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ���1��ʯ������
��2��������ʯ�����ƵIJ�Ʒ֮һ��ֱ����Ϊȼ��ʹ������ɻ�����Ⱦ���������м��������Ҵ����ɽ�ʡʯ����Դ����������β����Ⱦ��д���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽ
��3��ֻҪ���˶������̼Ҿ��ṩ��ѵ����ϴ��������ǵ������ṩ�˺ܶ�������������ڹ���ʹ�����Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⣬Ϊ�ˣ�����Ժ�칫������֪ͨ���ӽ����6��1�������еij��С��̳������м�ó�г��������ṩ��ѵ����ϴ���ij��ѧ�о�С���ͬѧ����ij�����Ϸ������������ʾ������ֻ��C��H����Ԫ�أ�����ɽ��вⶨ�����������ʵ�飬��ͼ��ʾ��
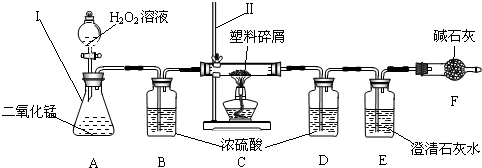
���������������
��װ��A���������ķ�Ӧ��1ѧ����ʽΪ
װ��E���������ķ�Ӧ��ѧ����ʽΪ
��װ��B��������
���о�С���ͬѧ��ʵ��ǰ����γ���װ��D����Ŀ����
��������C�IJ������з����������������ΪWg�������������ȼ�պ�������D����a g����Wg�����������к���Ԫ�ص�����Ϊ
����ʵ����û������װ��B���ⶨ��������������Ԫ�����������Ľ����
��������1��ʯ�����ڻ������������û�����������ɣ�
��2���Ҵ�ȼ��������ˮ�Ͷ�����̼��
��3�����ù������������ȡ������������̼�ܺ��������Ʒ�Ӧ����̼��ƺ�ˮ��Ũ���������ˮ�ԣ�����ˮ���������������������Ԫ�ص�������
��2���Ҵ�ȼ��������ˮ�Ͷ�����̼��
��3�����ù������������ȡ������������̼�ܺ��������Ʒ�Ӧ����̼��ƺ�ˮ��Ũ���������ˮ�ԣ�����ˮ���������������������Ԫ�ص�������
����⣺��1��ʯ�����ڻ���ʯ�ͷ������������仯���������������
��2���Ҵ�ȼ�յĻ�ѧ����ʽΪ��C2H5OH+3O2
2CO2+3H2O��
��3�������������������ƿ�������������������̨�������ƿ������̨��
��װ��A���������ķ�Ӧ�Ļ�ѧ����ʽΪ��2H2O2
2H2O+O2����
װ��E���������ķ�Ӧ��ѧ����ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O��
��װ��B�������Ǹ����������������������
���о�С���ͬѧ��ʵ��ǰ����γ���װ��D����Ŀ���Dz�ˮ�������������ˮ��������
��������D����a g��˵������ˮ��������ag����Wg�����������к���Ԫ�ص�����Ϊ��ag��
��100%=
g�����
��
����ʵ����û������װ��B����ⶨ��ˮ������ƫ�ⶨ��������������Ԫ�����������Ľ��Ҳ��ƫ���ƫ��
��2���Ҵ�ȼ�յĻ�ѧ����ʽΪ��C2H5OH+3O2
| ||
��3�������������������ƿ�������������������̨�������ƿ������̨��
��װ��A���������ķ�Ӧ�Ļ�ѧ����ʽΪ��2H2O2
| ||
װ��E���������ķ�Ӧ��ѧ����ʽΪ��CO2+Ca��OH��2�TCaCO3��+H2O��
��װ��B�������Ǹ����������������������
���о�С���ͬѧ��ʵ��ǰ����γ���װ��D����Ŀ���Dz�ˮ�������������ˮ��������
��������D����a g��˵������ˮ��������ag����Wg�����������к���Ԫ�ص�����Ϊ��ag��
| 2 |
| 18 |
| a |
| 9 |
| a |
| 9 |
����ʵ����û������װ��B����ⶨ��ˮ������ƫ�ⶨ��������������Ԫ�����������Ľ��Ҳ��ƫ���ƫ��
������������Ҫ������ط���ļ���ͻ�ѧ����ʽ����д�ȷ����֪ʶ����д��ѧ����ʽʱҪע����ѭ�����غ㶨�ɣ�

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
 �й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
�й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ������1��ʯ������
��2��������ʯ�����ƵIJ�Ʒ֮һ��ֱ����Ϊȼ��ʹ������ɻ�����Ⱦ���������м��������Ҵ����ɽ�ʡʯ����Դ����������β����Ⱦ��д���Ҵ��ڿ�����ȼ�յĻ�ѧ����ʽ��
��3��ʯ�����ƵIJ�Ʒ֮һʯ����������������
Ϊ�˲ⶨ������̼��������Ԫ�ص������ȣ�ij��ѧ��ȤС�����������ͼ��ʾ��ʵ�飮ʵ�鲽�����£��ȷֱ��������װ�â�װ�â����������ͼʾ���Ӻ�����װ�ã���ȼ����ͬʱ��a���ܿڳ�����һ��ʱ���Ϩ�������ٷֱ��������װ�â�װ�â��������ʵ�����ݼ��±��������ԭ��������H-1 C-12 O-16��
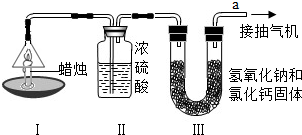
| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
���ɸ�ʵ�����ݼ��㣬������̼����Ԫ�ص�����֮��Ϊ
��װ�â��װ�â��˳���ܷ�ߵ���
�������ϣ�װ�â��װ�â����ӵ���������������ʧȥ����������ԭ����
�ݸ�ʵ���ܷ�ȷ�������ȼ�����ɶ�����̼��ˮ��������
 ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�
ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�
