��Ŀ����
���ҡ�ʮ����滮��ȷ�����衰��Լ��Դ�������������Ļ����Ѻ�����ᣬ�������Ի����Ѻ�������������ʶ���ش��������⣺��1�����������������磬�����������뷢�糵��ǰ����Ҫ��ȥһЩ����ȼ�յ����ʣ����������е����ʱ���ȥ������ ��
A���鲣�� B����ֽм C���ɵ�� D��ʳƷ������
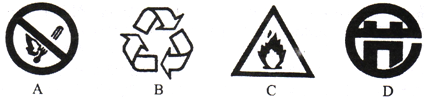
��2�������ǷŴ��ط�����Դ�����и�ͼ��������Ʒ���ձ�־���� ��

��3������������������������������Ҫ�ɷֺ���Ȼ����ͬ��������ȼ�տ����ڹ��Ȼ磬����ȫȼ�յĻ�ѧ����ʽΪ�� ��
���𰸡���������1���������⡰��ȥһЩ����ȼ�յ����ʡ�����֪ѡȡ���Dz���ȼ�յ����ʣ�
��2������ͼ����ʾ��־�ĺ��弰��Ŀ��Ҫ����з����жϼ��ɣ�
��3���������⣬��������Ȼ������Ҫ�ɷ���ͬ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
����⣺��1���鲣���ɵ�ؾ��Dz���ȼ�յ����ʣ����Ա����ȥ��
��2��A��ͼ����ʾ��־�ǽ�ֹ�̻��־��B��ͼ����ʾ��־����Ʒ���ձ�־��C��ͼ����ʾ��־�ǵ��Ļ���---��ȼ���ʱ�־��D��ͼ����ʾ��־�ǽ��ܱ�־��
��3����������Ȼ������Ҫ�ɷ���ͬ������Ҫ�ɷ��Ǽ��飬ȼ��ʱ��Ӧ��Ϊ���顢������������Ϊ������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2 2H2O+CO2��
2H2O+CO2��
�ʴ�Ϊ����1��AC����2��B����3��CH4+2O2 2H2O+CO2��
2H2O+CO2��
�����������ѶȲ�����Ҫ������ͬѧ�����������ѧ��ѧ֪ʶ�������⡢���������ʵ�������������
��2������ͼ����ʾ��־�ĺ��弰��Ŀ��Ҫ����з����жϼ��ɣ�
��3���������⣬��������Ȼ������Ҫ�ɷ���ͬ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
����⣺��1���鲣���ɵ�ؾ��Dz���ȼ�յ����ʣ����Ա����ȥ��
��2��A��ͼ����ʾ��־�ǽ�ֹ�̻��־��B��ͼ����ʾ��־����Ʒ���ձ�־��C��ͼ����ʾ��־�ǵ��Ļ���---��ȼ���ʱ�־��D��ͼ����ʾ��־�ǽ��ܱ�־��
��3����������Ȼ������Ҫ�ɷ���ͬ������Ҫ�ɷ��Ǽ��飬ȼ��ʱ��Ӧ��Ϊ���顢������������Ϊ������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2
 2H2O+CO2��
2H2O+CO2���ʴ�Ϊ����1��AC����2��B����3��CH4+2O2
 2H2O+CO2��
2H2O+CO2�������������ѶȲ�����Ҫ������ͬѧ�����������ѧ��ѧ֪ʶ�������⡢���������ʵ�������������

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ