��Ŀ����
�����Ǻ��������õ����ͣ���ʡ�ġ�ʮ���塱�滮ȷ���ˡ���ɫ���á���չս�ԣ�����ѧ��Դ���ۺ����ý����ص㷢չ����֮һ��
��1����ˮ������Ŀǰ������ˮ��õķ�����
��2������
���ƴ��Σ���±ˮ����ȡ�����õĽᾧ������
�ڴ����ᴿ�������к�����ɳ������þ���Ȼ��Ƶ����ʣ�����������ˮ�����ˣ�Ȼ��ͨ�����²������ɵõ��ϴ����Ȼ��ƣ�a���ˣ�b�ӹ�����Ba��OH��2��Һ��c�����������d�ӹ�����Na2CO3��Һ��e��������ȷ�IJ���˳����
��3���Ƽ����ƴ��������ͼ���£�

�����ͼ�С�-�������ݣ���
��д����NaHCO3�Ƶ�Na2CO3�Ļ�ѧ����ʽ��
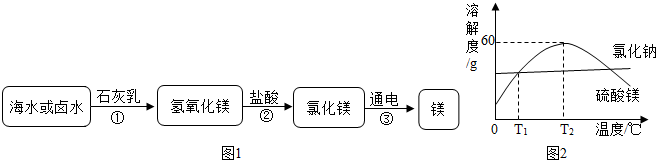
��4����þ��þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����Ҫ�������£�
��Ϊ��ʹMgCl2ת��ΪMg��OH��2���Լ��ٿ�ѡ��
���Լ��ڿ���ѡ��
��1����ˮ������Ŀǰ������ˮ��õķ�����
����
����
������2������
���ƴ��Σ���±ˮ����ȡ�����õĽᾧ������
�����ᾧ
�����ᾧ
���������ᾧ�����½ᾧ�������ڴ����ᴿ�������к�����ɳ������þ���Ȼ��Ƶ����ʣ�����������ˮ�����ˣ�Ȼ��ͨ�����²������ɵõ��ϴ����Ȼ��ƣ�a���ˣ�b�ӹ�����Ba��OH��2��Һ��c�����������d�ӹ�����Na2CO3��Һ��e��������ȷ�IJ���˳����
bdace
bdace
������ĸ������3���Ƽ����ƴ��������ͼ���£�

�����ͼ�С�-�������ݣ���
NH3
NH3
����CO2
CO2
��������
����
����д����NaHCO3�Ƶ�Na2CO3�Ļ�ѧ����ʽ��
2NaHCO3
Na2CO3+H2O+CO2��
| ||
2NaHCO3
Na2CO3+H2O+CO2��
��
| ||
��4����þ��þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����Ҫ�������£�

��Ϊ��ʹMgCl2ת��ΪMg��OH��2���Լ��ٿ�ѡ��
��������
��������
�������Լ��ٺ��ܹ�����õ�Mg��OH��2�����ķ���������
����
�����Լ��ڿ���ѡ��
����
����
����Ӧ�Ļ�ѧ����ʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O
Mg��OH��2+2HCl=MgCl2+2H2O
����������1����ˮ�����ķ�����Ҫ�У������ᾧ��������Ĥ�����༶���������ɸ���ԭ�����з�����
��2���ٿɸ����Ȼ����ܽ�����¶ȵĹ�ϵ���з���ѡ��ᾧ������
�ڸ��ݳ���ʱ�����������ʵ�ԭ����ʵ��Ŀ�Ľ��з�����
��3���������ʵĻ�ѧ���ʽ���ѡ���Լ���
��4�����Ȼ�þת��Ϊ������þ��Ҫ�����Һ��Ҫ������Һ�Ƿ�����ؼ��ǿ���Һ�Ƿ��Լ��ԣ����������Һ��һ����ù��˵ķ�ʽ���з��룮��������þת�����Ȼ�þһ���Dz�����������кͷ�Ӧ��
��2���ٿɸ����Ȼ����ܽ�����¶ȵĹ�ϵ���з���ѡ��ᾧ������
�ڸ��ݳ���ʱ�����������ʵ�ԭ����ʵ��Ŀ�Ľ��з�����
��3���������ʵĻ�ѧ���ʽ���ѡ���Լ���
��4�����Ȼ�þת��Ϊ������þ��Ҫ�����Һ��Ҫ������Һ�Ƿ�����ؼ��ǿ���Һ�Ƿ��Լ��ԣ����������Һ��һ����ù��˵ķ�ʽ���з��룮��������þת�����Ȼ�þһ���Dz�����������кͷ�Ӧ��
����⣺��1����������ˮ�����ú�ˮ�и��ɷݵķе�Ƚϸߣ�ˮ�ķе�Ƚϵͽ��з���ģ���ѹ����Ĥ��������ˮ�����ú�ˮ�и��ɷݵĿ�����С��ͬ��ˮ���ӿ���ͨ������Ĥ���������Ӳ���ͨ����
��2����Ϊ�Ȼ��Ƶ��ܽ�����¶ȵı仯�������Բ��������ܼ����ᾧ��
Ҫ��ȥ�����к��е����ʣ����Լӹ���������������Һ��ȥ����þ���ӹ�����Na2CO3��Һ��ȥ�Ȼ��ƺ����Լ�����������ͨ�����˰Ѳ�����ˮ�����ʳ�ȥ���������������ȥ������̼���ƣ�ͨ��������ȥ���ᣮ�ʴ�Ϊ�������ᾧ��bdace
��3����������������������з�����Ҫ�Ʊ����Ͱ�ˮ�϶��ǽ���ˮͨ��ʳ��ˮ�У��ɱ��͵İ�ˮʳ����Һ�Ʊ�̼�����ƣ��������ʵ����ʺ�Ԫ���غ�֪Ӧͨ�������̼���壻̼�����������ֽ��Ϊ̼���ƣ��仯ѧ����ʽΪ2NaHCO3
Na2CO3+H2O+CO2����
�ʴ�Ϊ����NH3��CO2�ۼ��ȣ�2NaHCO3
Na2CO3+H2O+CO2��
��4�����Ȼ�þת��Ϊ������þ��Ҫ�����Һ���ṩ���������˴�ѡ���������ƽ��з�Ӧ������������þ�dz����������Ƿ��������Һ��Ĺ��̣�һ����ù��˵ķ�ʽ���з��룮�ʴ�Ϊ���������ơ����ˣ��ڰ�������þת�����Ȼ�þ�������������кͷ�Ӧ���ʣ����ᣬMg��OH��2+2HCl=MgCl2+2H2O��
�ʴ�Ϊ����1������2���������ᾧ����bdace��
��3����NH3 ����CO2 ���ۼ��ȣ���2NaHCO3
Na2CO3+H2O+CO2����
��4�����������ƣ����ˣ������ᣬMg��OH��2+2HCl=MgCl2+2H2O��
��2����Ϊ�Ȼ��Ƶ��ܽ�����¶ȵı仯�������Բ��������ܼ����ᾧ��
Ҫ��ȥ�����к��е����ʣ����Լӹ���������������Һ��ȥ����þ���ӹ�����Na2CO3��Һ��ȥ�Ȼ��ƺ����Լ�����������ͨ�����˰Ѳ�����ˮ�����ʳ�ȥ���������������ȥ������̼���ƣ�ͨ��������ȥ���ᣮ�ʴ�Ϊ�������ᾧ��bdace
��3����������������������з�����Ҫ�Ʊ����Ͱ�ˮ�϶��ǽ���ˮͨ��ʳ��ˮ�У��ɱ��͵İ�ˮʳ����Һ�Ʊ�̼�����ƣ��������ʵ����ʺ�Ԫ���غ�֪Ӧͨ�������̼���壻̼�����������ֽ��Ϊ̼���ƣ��仯ѧ����ʽΪ2NaHCO3
| ||
�ʴ�Ϊ����NH3��CO2�ۼ��ȣ�2NaHCO3
| ||
��4�����Ȼ�þת��Ϊ������þ��Ҫ�����Һ���ṩ���������˴�ѡ���������ƽ��з�Ӧ������������þ�dz����������Ƿ��������Һ��Ĺ��̣�һ����ù��˵ķ�ʽ���з��룮�ʴ�Ϊ���������ơ����ˣ��ڰ�������þת�����Ȼ�þ�������������кͷ�Ӧ���ʣ����ᣬMg��OH��2+2HCl=MgCl2+2H2O��
�ʴ�Ϊ����1������2���������ᾧ����bdace��
��3����NH3 ����CO2 ���ۼ��ȣ���2NaHCO3
| ||
��4�����������ƣ����ˣ������ᣬMg��OH��2+2HCl=MgCl2+2H2O��
�����������ѶȱȽϴ��漰��ˮ����������ԭ������ˮɹ�ε�ԭ�������ӵ�ԭ���������Ƽ��ԭ�����������ݣ�Ҫͨ�����������ܽᣮ

��ϰ��ϵ�д�
 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ
 ��2012?���ˣ�21�����Ǻ��������õ����ͣ�
��2012?���ˣ�21�����Ǻ��������õ����ͣ�