��Ŀ����
��������ͷ�õĴ����к�������NaCl��ijʵ��С��Ҫ�ⶨ�ô�����Na2CO3������������ȡ6g������Ʒ�����ձ��У���μ���ϡ���������ٲ������ݣ���ʱ�ձ���û�в����������ϡ����61.7g����÷�Ӧ����Һ������Ϊ65.5g�������ʵ��С��������¼��㣺��1�����ɶ�����̼�������Ƕ��٣�
��2����Ʒ��̼���Ƶ����������Ƕ��٣����������0.1%����
��3����Ӧ����Һ�����ʵ����������Ƕ��٣�
���𰸡������������е��Ȼ����������Ӧ�����������������ɶ�����̼���壬����������Һ���������ˣ������ٵIJ���Ϊ������̼�����������ö�����̼�����������̼���Ƶ������ͷ�Ӧ����Һ�����ʵ�����������
����⣨1�����������غ㶨�����ɶ�����̼������Ϊ��6g+61.7g-65.5g=2.2g��
��2���裺��Ʒ�к�̼���Ƶ�����Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCL=2NaCl+H2O+CO2��
106 117 44
x y 2.2g

x= =5.3g
=5.3g
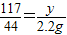
y= =5.85g
=5.85g
����Ʒ��̼���Ƶ���������Ϊ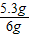 ×100%=88.3%
×100%=88.3%
��3����Ӧ����Һ�е����ʵ���������Ϊ ×100%=10%
×100%=10%
�����ɶ�����̼������Ϊ2.2g����Ʒ��̼���Ƶ���������Ϊ88.3%����Ӧ����Һ�е����ʵ���������Ϊ10%��
�������ڼ��㷴Ӧ����Һ�����ʵ���������ʱ��ѧ������ֻ��̼���������ᷴӦ���ɵ��Ȼ��Ƽ������ڣ���ԭ�����е��Ȼ��������ˣ������ڷ���ʱӦע��ԭ�����е��Ȼ��Ʊ�����룮
����⣨1�����������غ㶨�����ɶ�����̼������Ϊ��6g+61.7g-65.5g=2.2g��
��2���裺��Ʒ�к�̼���Ƶ�����Ϊx����Ӧ�����Ȼ��Ƶ�����Ϊy��
Na2CO3+2HCL=2NaCl+H2O+CO2��
106 117 44
x y 2.2g

x=
 =5.3g
=5.3g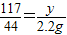
y=
 =5.85g
=5.85g����Ʒ��̼���Ƶ���������Ϊ
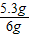 ×100%=88.3%
×100%=88.3%��3����Ӧ����Һ�е����ʵ���������Ϊ
 ×100%=10%
×100%=10%�����ɶ�����̼������Ϊ2.2g����Ʒ��̼���Ƶ���������Ϊ88.3%����Ӧ����Һ�е����ʵ���������Ϊ10%��
�������ڼ��㷴Ӧ����Һ�����ʵ���������ʱ��ѧ������ֻ��̼���������ᷴӦ���ɵ��Ȼ��Ƽ������ڣ���ԭ�����е��Ȼ��������ˣ������ڷ���ʱӦע��ԭ�����е��Ȼ��Ʊ�����룮

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ