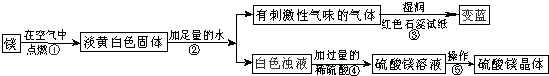
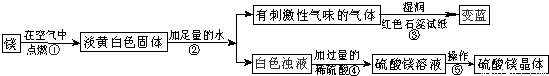
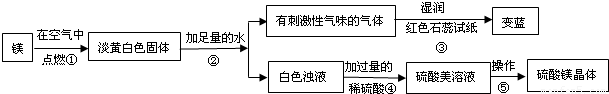
��Ŀ����
��ʦ����þ���ڿ�����ȼ�գ�ϸ�ĵ�ͬѧ���֣�ȼ�պ����������˰�ɫ��ĩ״�������⣬������������ɫ���壮ͬѧ�Dz�����֪������þ��ˮֻ������Ӧ���������ǶԵ��ư�ɫ������������µ�̽����
��ش����е����⣺
��1��þ�ڿ�����ȼ�յ������У�
��2���ɲ����۵�����˵����������
��3�������ݰ����ľ�����������ǣ�����Ũ��������
��������1������þ��ȼ��ʵ���֪��
��2����ɫʯ����ֽ������˵���̼��������ǰ�������д��ѧ����ʽҪע����������ƽ��
��3������þ��Һ��Ϊ����þ���壬���ǰ�ˮ���ɣ��������ᾧ��
��2����ɫʯ����ֽ������˵���̼��������ǰ�������д��ѧ����ʽҪע����������ƽ��
��3������þ��Һ��Ϊ����þ���壬���ǰ�ˮ���ɣ��������ᾧ��
����⣺��1���ɳ��е�þ��ȼ��ʵ���֪��ȼ��һ����淢�⡢�������ʴ�Ϊ������ҫ�۵İ⣻ �ų��������ȣ�
2Mg+O2
2MgO��
��2����ɫʯ����ֽ������˵���̼�����ζ�������ǰ�����������Ŀ��Ϣ��֪����Ӧ���ǹ���Mg3N2��ˮ���������ǰ�����������þ���ʴ�Ϊ��NH3�� Mg3N2+6H2O=3Mg��OH��2+2NH3����
��3������þ��Һ��Ϊ����þ���壬���ǰ�ˮ���ɣ��������ᾧ���ʴ�Ϊ���ᾧ��
2Mg+O2
| ||
��2����ɫʯ����ֽ������˵���̼�����ζ�������ǰ�����������Ŀ��Ϣ��֪����Ӧ���ǹ���Mg3N2��ˮ���������ǰ�����������þ���ʴ�Ϊ��NH3�� Mg3N2+6H2O=3Mg��OH��2+2NH3����
��3������þ��Һ��Ϊ����þ���壬���ǰ�ˮ���ɣ��������ᾧ���ʴ�Ϊ���ᾧ��
����������þ��ȼ�յ�ʵ������ס������ʹ��ɫʯ����ֽ������

��ϰ��ϵ�д�
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д� ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
�����Ŀ