��Ŀ����
��ʦ����þ���ڿ�����ȼ�գ�ϸ�ĵ�ͬѧ���֣�ȼ�պ����������˰�ɫ��ĩ״�������⣬������������ɫ���壮ͬѧ�Dz�����֪������þ��ˮֻ������Ӧ���������ǶԵ��ư�ɫ������������µ�̽����

��ش����е����⣺
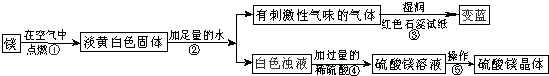
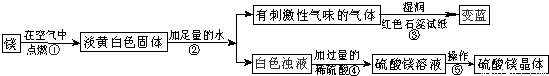
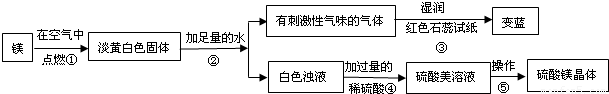
��1��þ�ڿ�����ȼ�յ������У�______���а�ɫ���̡�______���е��ư�ɫ�������ɣ�þ��������Ӧ�Ļ�ѧ����ʽΪ��______��
��2���ɲ����۵�����˵����������______���ѧʽ���������������˽������������þȼ�ղ����еĵ���ɫ����Mg3N2��ˮ��Ӧ����������þʱ���ɵģ��仯ѧ����ʽΪ��______��
��3�������ݰ����ľ�����������ǣ�����Ũ��������______��

��ش����е����⣺
��1��þ�ڿ�����ȼ�յ������У�______���а�ɫ���̡�______���е��ư�ɫ�������ɣ�þ��������Ӧ�Ļ�ѧ����ʽΪ��______��
��2���ɲ����۵�����˵����������______���ѧʽ���������������˽������������þȼ�ղ����еĵ���ɫ����Mg3N2��ˮ��Ӧ����������þʱ���ɵģ��仯ѧ����ʽΪ��______��
��3�������ݰ����ľ�����������ǣ�����Ũ��������______��
��1���ɳ��е�þ��ȼ��ʵ���֪��ȼ��һ����淢�⡢�������ʴ�Ϊ������ҫ�۵İ⣻ �ų��������ȣ�
2Mg+O2
2MgO��
��2����ɫʯ����ֽ������˵���̼�����ζ�������ǰ�����������Ŀ��Ϣ��֪����Ӧ���ǹ���Mg3N2��ˮ���������ǰ�����������þ���ʴ�Ϊ��NH3�� Mg3N2+6H2O=3Mg��OH��2+2NH3����
��3������þ��Һ��Ϊ����þ���壬���ǰ�ˮ���ɣ��������ᾧ���ʴ�Ϊ���ᾧ��
2Mg+O2
| ||
��2����ɫʯ����ֽ������˵���̼�����ζ�������ǰ�����������Ŀ��Ϣ��֪����Ӧ���ǹ���Mg3N2��ˮ���������ǰ�����������þ���ʴ�Ϊ��NH3�� Mg3N2+6H2O=3Mg��OH��2+2NH3����
��3������þ��Һ��Ϊ����þ���壬���ǰ�ˮ���ɣ��������ᾧ���ʴ�Ϊ���ᾧ��

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ