��Ŀ����
����Ŀ����ͼΪ��һ����̼����������Ӧ����ʵ��װ�ã� 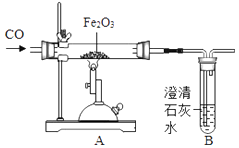
��1����ɸ�ʵ�飬�ܿ���A�е������� ��
��2��ʵ������У���װ��ĩ�˵ĵ��ܿڷ�һȼ�ŵľƾ��ƣ����洦�ڵ��ܿڣ�����������Ŀ���� ��
��3��ͨ��Bװ�ÿɼ���A�е������ﺬ��CO2 �� �÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4������ͨCO2�����ٽ��д�ʵ�飬ͨ���ԱȻ�ó�CO��CO2��ѧ���ʵIJ�ͬ�㣮����д�����е�һ�� �� ���Դ��۵ĽǶȷ���CO��CO2��ѧ���ʲ�ͬ��ԭ���� ��
��5��ij������ÿ��������4900t��Fe2O376%�ij�����ʯ������������ó������Ͽ��ղ���Fe 98%�������������������ݻ�ѧ����ʽ��ʽ���㣩
���𰸡�
��1����ɫ��ĩ���ɫ
��2����ȼβ���е�CO����ֹ��Ⱦ����
��3��CO2+Ca��OH��2=CaCO3��+H2O
��4��CO���п�ȼ�ԣ�CO2û�У����ӵĹ��ɲ�ͬ
��5���⣺�������Ͽ��ղ���Fe 98%������������Ϊx
Fe2O3+ | 3CO |
| 2Fe+3CO2 |
160 | 112 | ||
4900t��76% | x��98% |
![]() =
= ![]()
x=2660t
���������⣺��1��һ����̼���������ڸ��µ��������������Ͷ�����̼��������ɸ�ʵ�飬�ܿ���A�е������ǣ���ɫ��ĩ���ɫ����2��һ����̼�ж���Ҫβ��������������װ��ĩ�˵ĵ��ܿڷ�һȼ�ŵľƾ��ƣ����洦�ڵ��ܿڣ���Ŀ���ǣ���ȼβ���е�CO����ֹ��Ⱦ��������3���������ƺͶ�����̼��Ӧ����̼��Ƴ�����ˮ����ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O����4��CO��CO2��ѧ���ʵIJ�ͬ���ǣ�CO���п�ȼ�ԣ�CO2û�У����۵ĽǶȷ���CO��CO2��ѧ���ʲ�ͬ��ԭ���ǣ����ӹ��ɲ�ͬ�����Դ��ǣ���1����ɫ��ĩ���ɫ����2����ȼβ���е�CO����ֹ��Ⱦ��������3��CO2+Ca��OH��2=CaCO3��+H2O����4��CO���п�ȼ�ԣ�CO2û�У����ӵĹ��ɲ�ͬ����5��2660t��
�����㾫�������ڱ��⿼���һ����̼��ԭ����������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ����Ҫ�˽�ԭ�����ڸ����£����ý�̿��������Ӧ���ɵ�һ����̼����������ʯ�ﻹԭ������ע�⣺a����ƽ b������ c�����Ų��ܵó���ȷ�𰸣�