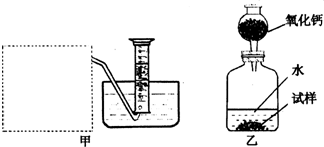
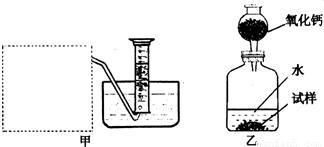
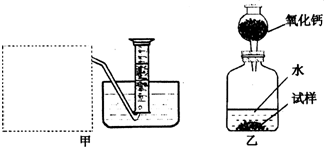
��Ŀ����
| |||||||||||||||||||
�𰸣�
������
������
(1) |
O2,�����ǵ�ľ�������������ܷ�ʹ�临ȼ |
(2) |
������̼��������������������ˮ�������ſ��� |
(3) |
���� |
(4) |
�����A |

��ϰ��ϵ�д�
�����Ŀ
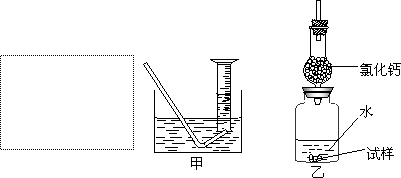

 �Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
�Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺