��Ŀ����
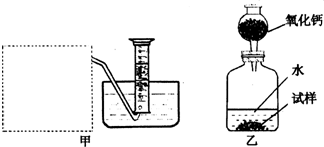
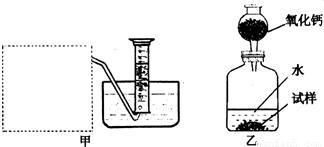
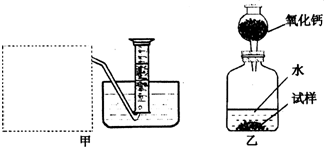 �Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
�Ƶ�������ͨ���������ƣ�Na2O���������ƣ�Na2O2����Na2O�ǰ�ɫ���壬Na2O2�ǵ���ɫ���壮Na2O2�г���������Na2O����ѧ��ȤС���ͬѧ���ⶨij������Na2O2�Ĵ��ȣ����������һ�����ʵ�飺
��1�������Ѿ�֪����Na2O+H2O�T2NaOH�����Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��2Na2O2+2H2O�T4NaOH+______����������������______��
��2������ȤС���ͬѧ����˼ס�������װ�ã���װ�������߷�����Ϊ���巢��װ�ã�����ΪӦ��ʵ������ȡ______��װ����ͬ������ȤС��ѡ�����ַ����ռ������������______������Ϊ��������______���ռ���
��3����װ�������ⶨ���������______��
��4����װ�������ⶨ���������______������״���£�����֪��������������Ҫ�����Na2O2�Ĵ��ȣ�����Ҫ֪����������______��
A�������ڱ�״���µ��ܶȡ���
B����Ӧװ���м���ˮ������
C����Ӧ����Һ��������
�⣺��1��Na2O2��H2O��Ӧ�����������ƺ���������Ϊ��������ȼ�ԣ���˿��ô����ǵ�ľ�������飬�ʴ�Ϊ��O2�������ǵ�ľ����
��2�����������ǹ��壬ˮ��Һ�壬��˴˷�Ӧ�ǹ�Һ�ڳ����·�Ӧ��ȡ���壬���Ժ�ʵ�����ù���������ȡ������ʵ�����ô���ʯ��ϡ������ȡ������̼�ķ���װ����ͬ��ͼ������ˮ���ռ�����֪������������ˮ����Ϊ�������ܶȱȿ�������˻����������ſ������ռ����ʴ�Ϊ�������������̼��������������ˮ�������ſ�����
��3��������װ�õ��ص��֪��װ�������ⶨ����������������ʴ�Ϊ��������
��4�����ݼ�װ�õ��ص��֪��װ�������ⶨ��������������Ҫ�����Na2O2�Ĵ��ȣ���֪��������������Ŀ����ͨ��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��ģ��Ѳ����������������ֻҪ֪���������ܶȼ��ɣ���˴�Ϊ�������A��
��������1�����ݹ������ƺ�ˮ�ķ�Ӧ���������������ļ��鷽��������
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã������������ռ�װ���ж��������ܶȺ�ˮ���ԣ�
��3��������װ�õ��ص��ж�����;��
��4�����ݼ�װ�õ��ص��ж�����;��Ҫ�����Na2O2�Ĵ��ȣ���֪��������������Ŀ����ͨ��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��ã��Ѳ����������������ֻҪ֪���������ܶȼ��ɣ�
������������Ҫ����ѧ�������Ƶ��������������������Լ�����ˮ�������ⶨ���������ķ������ѶȲ���
��2�����������ǹ��壬ˮ��Һ�壬��˴˷�Ӧ�ǹ�Һ�ڳ����·�Ӧ��ȡ���壬���Ժ�ʵ�����ù���������ȡ������ʵ�����ô���ʯ��ϡ������ȡ������̼�ķ���װ����ͬ��ͼ������ˮ���ռ�����֪������������ˮ����Ϊ�������ܶȱȿ�������˻����������ſ������ռ����ʴ�Ϊ�������������̼��������������ˮ�������ſ�����
��3��������װ�õ��ص��֪��װ�������ⶨ����������������ʴ�Ϊ��������
��4�����ݼ�װ�õ��ص��֪��װ�������ⶨ��������������Ҫ�����Na2O2�Ĵ��ȣ���֪��������������Ŀ����ͨ��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��ģ��Ѳ����������������ֻҪ֪���������ܶȼ��ɣ���˴�Ϊ�������A��
��������1�����ݹ������ƺ�ˮ�ķ�Ӧ���������������ļ��鷽��������
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã������������ռ�װ���ж��������ܶȺ�ˮ���ԣ�
��3��������װ�õ��ص��ж�����;��
��4�����ݼ�װ�õ��ص��ж�����;��Ҫ�����Na2O2�Ĵ��ȣ���֪��������������Ŀ����ͨ��Na2O2��H2O��Ӧ�Ļ�ѧ����ʽ��ã��Ѳ����������������ֻҪ֪���������ܶȼ��ɣ�
������������Ҫ����ѧ�������Ƶ��������������������Լ�����ˮ�������ⶨ���������ķ������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ