��Ŀ����
��2004?���ݣ�ijͬѧȥ���еĵ���ɽ�羰������ʱ��ȡ�������ɿ��ʯ��Ʒ�������������µķ�������Ʒ��̼��Ƶ������������м�⣺ȡ����ʯ��ʯ��Ʒ6�ˣ���40��ϡ������Ĵμ��룬���������������ݼ��±�����֪ʯ��ʯ��Ʒ�к��е����ʲ�����ˮ���������ᷴӦ��������1��6��ʯ��ʯ��Ʒ�к��е�����Ϊ______�ˣ�
��2��m=______��
��3����Ʒ��̼��Ƶ�����������______��
��4��
| ����ϡ����Ĵ��� | 1 | 2 | 3 | 4 |
| ����ϡ������������ˣ� | lO | 10 | lO | lO |
| ʣ�������������ˣ� | 4.0 | m | O��6 | 0.6 |
���𰸡���������1���Ƚϵ����κ͵��Ĵε����ݿ��Եó���Ʒ�����ʵ�����Ϊ��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪��һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m��ֵ��
��3�������ʵ������������̼��Ƶ��������������̼��Ƶ�����������
��4�������������֪����һ�������2g̼���ǡ����ȫ��Ӧ�����Ծݴ�������������������
����⣺��1���Ƚϵ����κ͵��Ĵε����ݿ�֪����Ʒ�����ʵ�����Ϊ0.6g��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪����һ�μ���10g�����ʣ����������Ϊ4.0g���������μ���ϡ�����ʣ����������Ϊ0.6g�����Կ����жϵ�һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����10g ϡ�����ܹ�����2g̼��ƣ���˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m=4.0-2.0=2.0��
��3��̼��Ƶ�����Ϊ��6.0g-0.6g=5.4g��������������Ϊ��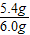 ×100%=90%
×100%=90%
��4�������������ΪX����
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
2g X

X=1.46 g��
�����������������Ϊ��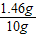 ×100%=14.6%��
×100%=14.6%��
�ʴ�Ϊ����1��0.6��
��2��2.0��
��3��90%��
��4��14.6%��
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м���������������ʱѧ����Ҫ�������ͼ�����ݣ��������ʷ�Ӧʱ��������ϵ����ȷ���ù�ʽ�ͻ�ѧ����ʽ���н��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪��һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m��ֵ��
��3�������ʵ������������̼��Ƶ��������������̼��Ƶ�����������
��4�������������֪����һ�������2g̼���ǡ����ȫ��Ӧ�����Ծݴ�������������������
����⣺��1���Ƚϵ����κ͵��Ĵε����ݿ�֪����Ʒ�����ʵ�����Ϊ0.6g��
��2���Ƚϵ�һ�κ͵����ε����ݿ�֪����һ�μ���10g�����ʣ����������Ϊ4.0g���������μ���ϡ�����ʣ����������Ϊ0.6g�����Կ����жϵ�һ����������ȫ��Ӧ������̼���6.0g-4.0=2.0g����10g ϡ�����ܹ�����2g̼��ƣ���˵ڶ�����Ҳ������2.0g̼��ƣ��ʿ������m=4.0-2.0=2.0��
��3��̼��Ƶ�����Ϊ��6.0g-0.6g=5.4g��������������Ϊ��
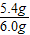 ×100%=90%
×100%=90%��4�������������ΪX����
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
2g X

X=1.46 g��
�������������������
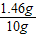 ×100%=14.6%��
×100%=14.6%���ʴ�Ϊ����1��0.6��
��2��2.0��
��3��90%��
��4��14.6%��
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м���������������ʱѧ����Ҫ�������ͼ�����ݣ��������ʷ�Ӧʱ��������ϵ����ȷ���ù�ʽ�ͻ�ѧ����ʽ���н��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
