��Ŀ����
����Ŀ���ҹ�����ǧ��ǰ���̴���������˾�������ͭ������ͭ�������õ������ԡ���ĥ�Ժ���ʴ�ԡ���ش��������⣺
��1���������������ͭ��ʱ�����̶���������ʱ��������������������ʷ��100���꣬����Ⱥ�˳������������йص�����_______��
A�����Ļ��˳�� B�����ĵ��硢������
C��������չ�� D�ؿ��н���Ԫ�صĺ���
��2����ͭ��ͭ���Ͻ�������Ӧ���������칤�������������������������صĻ�ѧ����ʽ�ɱ�ʾΪ��SnO2+2C![]() Sn+2CO������÷�Ӧ�Ļ�������Ϊ____________��
Sn+2CO������÷�Ӧ�Ļ�������Ϊ____________��
��3��ȡ��ͭ��Ʒ8.1g��������֪���к���0.9g�������ͭ��ͭ������������Ϊ________��
��4�����û�ѧ����������ͭ�ʹ�ͭ��д�����еIJ���������ͽ��ۣ�________________��
���𰸡�A �û���Ӧ 8:1 ����ͭ�ʹ�ͭƬ�Ϸֱ�μ�ϡ���ᣬ�����ݲ���������ͭ��û�����ݲ������Ǵ�ͭ
��������
��1���������ģ��ʹ�õ��Ⱥ�˳��������Ļ���йأ�������Խ���ã�Խ��ұ���������õ�Խ������ѡA��
��2������SnO2+2C![]() Sn+2CO�����÷�Ӧ�ķ�Ӧ����һ�ֵ��ʺ�һ�ֻ��������������һ�ֵ��ʺ���һ�ֻ���������û���Ӧ��
Sn+2CO�����÷�Ӧ�ķ�Ӧ����һ�ֵ��ʺ�һ�ֻ��������������һ�ֵ��ʺ���һ�ֻ���������û���Ӧ��
��3����ͭ��ͭ���Ͻ��������֪������ͭ�к�ͭ������Ϊ��8.1g-0.9g=7.2g�������ͭ��ͭ�������������ǣ�7.2g��0.9g=8��1��
��4����ͭ��ͭ���Ͻ����ڽ������˳�����������ǰ�棬�����ᷴӦ������������ͭ���ܡ��û�ѧ����������ͭ�ʹ�ͭ�����еIJ���������ͽ��ۣ�����ͭ�ʹ�ͭƬ�Ϸֱ�μ�ϡ���ᣬ�����ݲ���������ͭ��û�����ݲ������Ǵ�ͭ��

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�����Ŀ����������ʵ�����ݺ��ܽ�����ش�����С�⡣
�� | �� | �� | �� | |
�¶� | 10�� | 10�� | 30�� | 30�� |
�������� | KNO3 | NaCl | KNO3 | NaCl |
�������� | 30g | 30g | 40g | 40g |
ˮ������ | 100g | 100g | 100g | 100g |
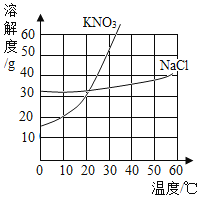
��1����~�����õ���Һ�У����ڲ�������Һ���ǣ�������
A.�ٺ͢�B.�ں͢�C.�ۺ͢�D.�ٺ͢�
��2������˵���У���ȷ���ǣ�������
A.������������=��B.��Һ�������ڣ���
C.�������ܼ������ȣ���=��D.���������������ۣ���