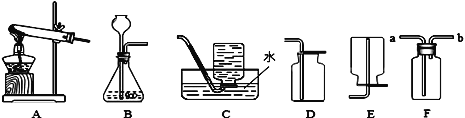
��Ŀ����
����Ŀ��A��F�dz��л�ѧ�г��������ʣ�����֮��Ĺ�ϵ����ͼ��ʾ ����������ʾת����ϵ����������ʾ�����Ӧ����A�Dzμ�ֲ�������õijɷ�֮һ��E������θҺ�е�һ���ᣬB��D��F�������ֲ�ͬ���͵����ʣ�����B��D������Ԫ�أ�F�dz��õĹ�������������ش��������⣺
��1��F���ʵ�������__________��
��2��D��E��Ӧ�Ļ�ѧ����ʽ��_______________________��
��3��B��C��Ӧ�Ļ�ѧ����ʽ��________________________��
���𰸡� ��ʯ�� NaOH+HCl=NaCl+H2O Na2CO3+Ca(OH)2=2NaOH+CaCO3��
�����������������ƶ��⣬����Ĺؼ����������ʵ������ж����ʣ���ͨ�����ת����ϵ���������̽��з������Ӷ��õ���ȷ�Ľ��ۡ������е�����������A��E��F��
������Ŀ����������ͼ����Ϣ��A�Dzμ�ֲ�������õijɷ�֮һ�� A�����Ƕ�����̼��E������θҺ�е�һ�ֳɷ֣� E�����A��F�dz��л�ѧ�г��������ʣ�B�к�����Ԫ�أ�B����������̼��B������̼���ƣ�D�к�����Ԫ�أ�B��D���ڲ�ͬ���͵����ʣ�D��ת��Ϊ̼���ƣ����������������̼��Ӧ����̼���ƺ�ˮ��D�������������ƣ�F�dz��õĹ�����������F�����������ƣ���������ת��ΪC��������̼��C������Ӧ����������ˮ��Ӧ�����������ƣ�������̼�����������Ʒ�Ӧ����̼��ƺ�ˮ����C���������ƣ�����������ϵͼ�������������ˣ�
��1��F�������ƣ��׳���ʯ�ң�
��2��D���������ƣ�E�����ᣬ�������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��NaOH+HCl=NaCl+H2O��
��3��B��̼���ƣ�C���������ƣ�̼���ƺ��������Ʒ�Ӧ����̼��ƺ��������ƣ���Ӧ�Ļ�ѧ����ʽ��Na2CO3+Ca(OH)2=2NaOH+CaCO3����

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�����Ŀ�������仯����Ӧ�ù㷺��
(һ)�����仯�����Ӧ��
����ͼ3ΪijС�����������ǽ���
(1)�ʵ����ø��ǽ�������______________(ѡ�����)��
A�����Ͳ� B��٪��֢ C��ƶѪ֢
(2)ͼ�����漰���IJ�����_________________(ѡ�����)��
A�������� B���ϳɲ��� C�����ϲ���
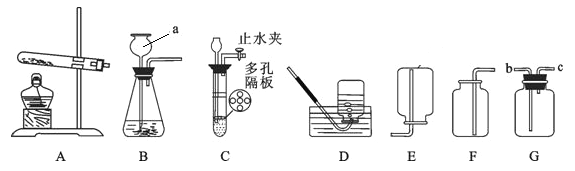
��ʳƷ������˫�����������ۡ�ʳ�εȣ���ͼ4Ϊ��ԭ��̽��ʵ�飺
(1)����ʳ��ˮ�رշ�Һ©��������һ��ʱ��������ϳ��ֺ�ɫ���壬����______(ѡ�������������������������С��)��
(2)ʳ��ˮ��������______��
(��)�����仯�����ʵ��
(1)������м�������ϴ�Ӽ�����ˮ�У���ֽ����Գ�ȥ���ۣ���������ϴ�Ӽ���______���á�
(2)ȡ�����Ĺ�����м����һ������ϡH2SO4(ˮԡ���ȿ�����50��80��)�����衢���á����ˡ�ϴ�ӣ�����������ϴ���ķ�����________��
(3)������������Һ�м��뱥��(NH4)2SO4��Һ��������Ũ����________�����˵Ȳ����õ�dz����ɫ������
���������ϣ���������Ԫ�ص�dz����ɫ�����У���������������[FeSO47H2O]��
����������茶���[FeSO4(NH4)2SO46H2O]��
(4)ȡһ����������Ʒ����������ˮ������������______��Һ���ȣ����Ѳ���������ͨ���̪��Һ����̪��Һ���______ɫ��˵���þ�������������茶��塣
(��)��������茶�����ȷֽ�ʵ��
��ȤС���ȡ�˾�����Ʒ39.2g���ڿ�����Ա��ָ��������ͼװ�ý����ȷֽ�ʵ�顣
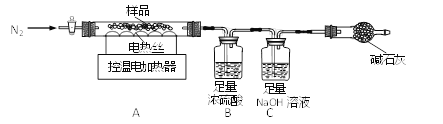
����������������������茶���(��Է�������Ϊ392)��100����ȫʧȥ�ᾧˮ��ʣ������500����ȫ�ֽ�Ϊ����ij�����SO2��SO3��NH3��H2O��
��Bװ����Ũ����ֻ������NH3��SO3��H2O��
(1)װ���ʵ��װ�ú���Ҫ________��
(2)����ǰ�����о�ͨ��N2��ֹͣ���Ⱥ����ͨN2��Ŀ���Ƿ�ֹ������________��
(3)���Ʋ�ͬ���¶ȶ�A�й�����ȣ����װ��B��C�е������仯���±���
�¶�/�� | ���� | 100 | 500 |
Bװ��/g | 200.00 | x | 228.00 |
Cװ��/g | 100.00 | 100.00 | 103.20 |
�ٱ�����x =________��
����������SO3������Ϊ________g������ij������Ļ�ѧʽΪ________��
����Ŀ���±��Dz�ͬ�¶�ʱ����ص��ܽ�ȣ��й�˵����ȷ���ǣ��� ����
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ��/�� | 13.3 | 31.6 | 63.9 | 110 | 169 | 246 |
A. 20��ʱ��100������ر�����Һ�к������31.6��
B. 40��ʱ����ر�����Һ�У���������ص���������Ϊ63.9%
C. 100���ˮ���ܽ�������һ����80���ˮ���ܽ������ض�
D. 100��ʱ��100+246��������ر�����Һ���µ�60��ʱ����������ع��������Ϊ136��
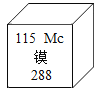
����Ŀ��������ճ��ر���Ʒ֮һ������Ҳ�ǻ�����Ⱦ��һ����Ҫ��Դ��������ij��ȤС�����÷Ͼ�п�̸ɵ����Ϊԭ�ϣ����������̽���Ĺ��̡�
��֪ʶ������
(1)п�̵�صĹ������ɣ���ͼ����

(2)�ᾧˮ������������ʣ����������¶������£��ᾧˮ������ʧȥ���ֻ���ȫ���ᾧˮ������ɫ�ĵ������壨CuSO4 5H2O������ʱ��ʧȥ�ᾧˮ��Ϊ��ɫ����ˮ����ͭ��ĩ��CuSO4����
I.�Ʊ�𩷯���壨ZnSO4xH2O��
С��ͬѧ�ι���ij���շϾ�п�̵�صĹ���������չ���������ͼ��

(1)����ͼ���Լ�a�Ļ�ѧʽ��________���õ�����Һ1ũҵ�Ͽ�����_______________��
(2)������B�ڿ����г�����տ��ᴿ�ƵõĹ�����_________���÷����ᴿ��ԭ���ǣ��û�ѧ����ʽ�ش�______________________��
��3���ⶨпƤ��ͭо����п��������������ȡ��ͬ����пƤ��ͭо�����ձ��У��������Ũ�ȵ�ϡ���ᣬ�������±���ʾ��
пƤ��ͭо�������� | 20g | 15g |
ϡ��������� | 100g | 120 g |
������������� | 0.4 g | 0.4 g |
��пƤ��ͭо����п��������������д��������̣��𰸱���һλС����_______
(4)��Һ2��������Ҫ������п�����й��ܽ�Ⱥ��¶ȹ�ϵ���±���
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
����Һ2����Ũ����_____________�����ˡ�ϴ�ӡ�����ɵõ�𩷯���壨ZnSO4xH2O)��
С��ͬѧ������𩷯���壨ZnSO4xH2O������ʵ���ң�����ͼװ�òⶨ�����нᾧˮ�ĺ�����ͼ�а�Ĥ��������ͨ���ֿɷ�ֹ�����ĩ���뵼�ܣ����ⶨ��������ȡ28.7g��������Cװ�õ�Ӳ�ʲ������У���������ȫʧȥ�ᾧˮ:

��ZnSO4xH2O ==== ZnSO4 + xH2O������ȴ�����º����������й���������Ϊ16.1g��
��5�� A�еĻ�ѧ����ʽ��______________��B�е��Լ��ɴ�����������ѡȡ�����ѡ����________��
A.Ũ���� B.��������Һ C.����̼������Һ D.����ʯ��ˮ
��6��ʵ�����������ͨ�������õĽ����____________
���ƫ�� ��ƫС������Ӱ�족��������ʵ����������𩷯�����нᾧˮ��xֵΪ_____��
��7��������𩷯������Ȼ���ʧȥ���ֽᾧˮ�����ȹ������йز���������������ͼ��д��C-D
�η�����Ӧ�Ļ�ѧ����ʽ____________________��

����Ŀ����7�֣�ijͬѧ��ʵ���ҷ�����һƿ��ǩ��ȱ����ɫ��Һ�v��ͼ����ʾ�w��Ϊȷ�����е����ʣ�����Ʋ�����������̽�������ش��������⡣
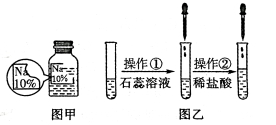
����������衿�����ʿ���ΪNaCl��NaOH��Na2CO3��NaHCO3�е�һ�֡�
�����ϲ��ġ������������ʵ������Ϣ���£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ�ȣ�g | 36 | 109 | 21.5 | 9.6 |
������ijϡ��Һ��pH | 7 | 13 | 11 | 9 |
��̽�����̡�
��ͼ����ʾ���ڲ����ٺ��ȷ�����ʲ���NaCl������ʵ������Ӧ�� ��
�ڽ��в�����ʱ����ɫ��ζ�õ�����������ɴ��ֿ��ų����������е� ��
��̽�����ۡ�
����Ϊ����Һ�е����ʿ������������������е� ������ж������� ��
��̽����˼��
��1��������̽����������ȷ�ģ������ڲ���������Ӧ�� ��д��ѧʽ����ʵ���Ҽ���������ʵ������������� ��
��2������ͬѧ�������е����ʻ�������Na2SO4������û��Na2SO4������������Ϣ��
�����������ʵ���������������жϸ���Һ�е������Ƿ���Na2SO4�����������ɣ� ��