��Ŀ����
��4�֣������벻����ѧ����ѧ������ϢϢ��ء�
��1������ʳ����ҪΪ�����ṩ������� ������ţ���



A���߲� B������ C������
��2������������Ʒ��ʹ�õIJ����У��������л��ϳɲ��ϵ��� ������ţ���

A.����ë�� B.�������� C.�ϳ���Ь�� D.��������
��3��ũ������ƹ�ʹ����������Ҫ�ɷ�Ϊ���飩ȼ�շ��磬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��ú��Ϊȼ�ϸ������ṩ������ͬʱ������һЩ�Ի��������Ⱦ�����ʣ����л��γ������������ ������ţ���
A. CO2 B.CO C. SO2 D. NO2
��1������ʳ����ҪΪ�����ṩ������� ������ţ���



A���߲� B������ C������
��2������������Ʒ��ʹ�õIJ����У��������л��ϳɲ��ϵ��� ������ţ���

A.����ë�� B.�������� C.�ϳ���Ь�� D.��������
��3��ũ������ƹ�ʹ����������Ҫ�ɷ�Ϊ���飩ȼ�շ��磬�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4��ú��Ϊȼ�ϸ������ṩ������ͬʱ������һЩ�Ի��������Ⱦ�����ʣ����л��γ������������ ������ţ���
A. CO2 B.CO C. SO2 D. NO2
��1��C ��2��A ��3�� CH4+2O2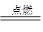 CO2+2H2O ��4��C D
CO2+2H2O ��4��C D
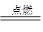 CO2+2H2O ��4��C D
CO2+2H2O ��4��C D �����������1������ʳ���еĴ�������ҪΪ�����ṩ����ģ�ѡC��
��2������ë�����õ����������л��ϳɲ��ϣ����ϡ������������л��ϳɲ��ϡ�ѡA��
��3������ȼ�յĻ�ѧ����ʽΪCH4+2O2
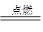 CO2+2H2O��
CO2+2H2O����4��SO2��NO2�ǻ��γ���������塣ѡCD��
������ʳ���е�Ӫ���������ࡢ�����ʡ���֬��ˮ�����κ�ά���أ�
�ޡ��顢��˿������Ȼ��ά��
��д��ѧ����ʽҪ��ѭ����ʵ�������غ㶨������ԭ��ע�⻯ѧʽҪ��ȷ����Ҫ���Ƿ�Ӧ������������߳������š�
SO2��NO2�ǻ��γ���������塣

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
�����Ŀ