��Ŀ����
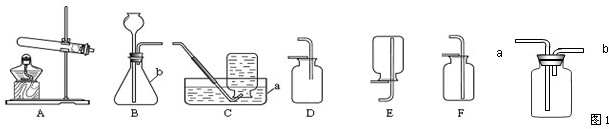
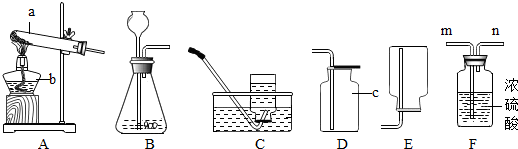
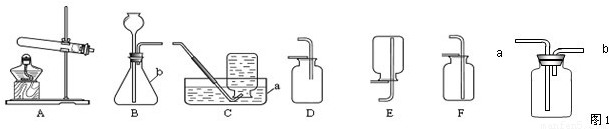
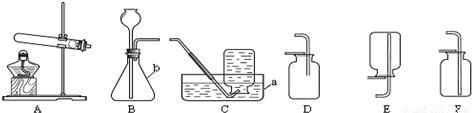
ʵ������ȡ��������װ������ͼ��ʾ��

A B C D E
��ش��������⣺
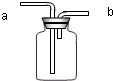
��1��װ���бꡰa����b�����������Ʒֱ���_____ �� ��
��2������װ��A��C������ȡ���������ռ���������Ӧ�� ���� ��
��3����װ��B�� ���ӿ�����ȡ������̼�����װ��B�����Եķ�����____ .
��4��ʵ���ҿ�������ˮ�����ƺͼ�ʯ�����ֹ�̬ҩƷ������ĥ���Ȼ�Ϻ�װ�뷴Ӧװ���У����Ȳ����������塣��ʵ���ѡ�õ����巢��װ��Ϊ ������ĸ��ţ���������
��5����25gʯ��ʯ��Ʒ����������ϡ�����У�ʯ��ʯ�е�̼���������ǡ����ȫ��Ӧ�����ʲ���Ӧ��Ҳ���ܽ⣩���������������Ϊ8.8g����ʯ��ʯ��̼��Ƶ���������Ϊ���٣�
(1) ��ƿ ����ƿ ��2�֣�
(2) �ѵ����ܴ�ˮ�����ó� Ϩ��ƾ��� ��2�֣�
(3) D��1�֣� �õ��ɼм�ס�ܣ���©����ע��ˮ��������©�����γ�һ���ȶ���ˮ����˵��װ�ò�©����2�֣�
(4) A ��1�֣� ��Ӧ���ǹ��壬��Ӧ����ȣ�2�֣�
(5) ��6�֣�
�⣺��μӷ�Ӧ̼��Ƶ�����Ϊx
CaCO3��2HCl��CaCl2��H2O��CO2��
100 44
x 8.8 g
100 :44=x: 8.8 g
x= 20g
ʯ��ʯ��̼��Ƶ���������Ϊ��
20g/25g��100�� =80��
��ʯ��ʯ��̼��Ƶ���������Ϊ80��
����:��