��Ŀ����
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��
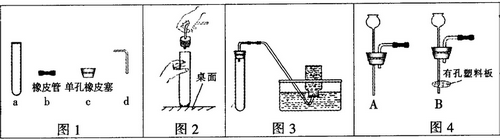
��1������ͼ1�ش�д��a������ ��������c��dʱ��ʹd���ײ���c�еĴ�ʩ�� ���� ��
��2������ͼ2��ʾ����������ɵĺ��֮һ�� ��
��3������ͼ3װ��(�г�װ��δ����)�ܽ��е�����ʵ���� ������ĸ����
д������ѡȫ���𰸵Ļ�ѧ����ʽ ��
����ͼ3�е�ˮ��Ϊ��ˮ������ѡ���л����Կ�������һ��������������ĸ���������� ������������������������������ ��
��4��ͼ3�е����巢��װ����Ȼ���������㣬�������Ʒ�Ӧ���ʡ����ͼ4��ѡȡ (����ĸ)ֻ��һ���Ľ���������ͼ1��a��װ���µ����巢��װ�ã��Դﵽ���Ʒ�Ӧ���ʵ�Ŀ�ģ���Ľ��ķ�����������������������������������

��1������ͼ1�ش�д��a������ ��������c��dʱ��ʹd���ײ���c�еĴ�ʩ�� ���� ��
��2������ͼ2��ʾ����������ɵĺ��֮һ�� ��
��3������ͼ3װ��(�г�װ��δ����)�ܽ��е�����ʵ���� ������ĸ����
| A���ø������������ | B����ʯ��ʯ��ϡ�����ƶ�����̼ |
| C����п��ϡ���������� | D����˫��ˮ��������������� |
����ͼ3�е�ˮ��Ϊ��ˮ������ѡ���л����Կ�������һ��������������ĸ���������� ������������������������������ ��
��4��ͼ3�е����巢��װ����Ȼ���������㣬�������Ʒ�Ӧ���ʡ����ͼ4��ѡȡ (����ĸ)ֻ��һ���Ľ���������ͼ1��a��װ���µ����巢��װ�ã��Դﵽ���Ʒ�Ӧ���ʵ�Ŀ�ģ���Ľ��ķ�����������������������������������
(1) �Թ� ��������Ƥ�ܵĵ���һ����ˮʪ�� (2) ѹ���Թܣ�������֣���
(3) C��D��ȫ�Ը�1�֣���
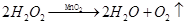 Zn + H2SO4 ="=" ZnSO4 + H2��
Zn + H2SO4 ="=" ZnSO4 + H2��
�¡��¶����ߣ�������ˮ�е��ܽ�ȼ�С��
(4) �¡�������©����Ϊ��Һ©��
(3) C��D��ȫ�Ը�1�֣���
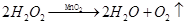 Zn + H2SO4 ="=" ZnSO4 + H2��
Zn + H2SO4 ="=" ZnSO4 + H2���¡��¶����ߣ�������ˮ�е��ܽ�ȼ�С��
(4) �¡�������©����Ϊ��Һ©��
���������(1) a������Ϊ�Թܣ�������c��dʱ��ʹd���ײ���c�еĴ�ʩ�ǽ�������Ƥ�ܵĵ���һ����ˮʪ��(2) ����ͼ2��ʾ�����ܿ��ܻ�ѹ���Թܣ�(3)ͼ3װ�ò���Ҫ���ȣ���Ӧ����Һ�壬�ռ�װ��Ϊ��ˮ�������Կ�������п��ϡ������������˫��ˮ�����������������ʵ��װ�ã�����ʽΪ
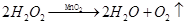 ��Zn + H2SO4 ="=" ZnSO4 + H2�����ռ�װ��������ˮ���������̼�����е��ܽ�Ƚ�С�����Բ�����ˮ���ռ����������װ��Ҳ����������ȡ������̼��(4) ���ã�װ�ý�������©����Ϊ��Һ©�����Դﵽ���Ʒ�Ӧ���ʵ�Ŀ�ġ�
��Zn + H2SO4 ="=" ZnSO4 + H2�����ռ�װ��������ˮ���������̼�����е��ܽ�Ƚ�С�����Բ�����ˮ���ռ����������װ��Ҳ����������ȡ������̼��(4) ���ã�װ�ý�������©����Ϊ��Һ©�����Դﵽ���Ʒ�Ӧ���ʵ�Ŀ�ġ�����������ʵ���ҵĻ�������ʹ�úͳ����������ȡ�����п��ıؿ����ͣ����ǿ��Կ��IJ���������Ƚ����ޣ�Ӧ�μǻ���֪ʶ�㣬ע��ϸ�ġ�

��ϰ��ϵ�д�
�����Ŀ





 �� ʵ���ҳ����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3����������NH3��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С����������ˮ���������ر���ճĤ��֯�и�ʴ���á�
�� ʵ���ҳ����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3����������NH3��һ����ɫ���д̼�����ζ�����壬�ܶȱȿ���С����������ˮ���������ر���ճĤ��֯�и�ʴ���á�
