��Ŀ����
����Ŀ��2018��5��18���ҹ���һ�ҹ�����ĸ�Ժ��ɹ�����ĸ���������Ԫ��ʹ���˻�ͭ��Ϊ�ⶨij��ͭ������Ͻ��н���ͭ��п����ͭ��������������ȤС��ͬѧ��ȡ20g��ͭ��ĩ���ձ��У���80gϡ������Ĵμ��룬��ַ�Ӧ�����ʵ���������±���ʾ��
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
����ϡ�����������g�� | 20 | 20 | 20 | 20 |
�ձ���ʣ�����������g�� | 39.92 | 59.84 | 79.80 | 99.80 |
��1����ͭ��ĩ��ȫ��Ӧ����������������Ϊ____________g��
��2���û�ͭ��ͭ����������Ϊ���٣���д��������̣�____________
���𰸡�0.2 67.5%�����������
��������
��1�����������غ㶨�ɿɵã���ͭ��ĩ��ȫ��Ӧ��������������=20g+80g-99.80g=0.2g
��2���裺20�˻�ͭ��ͭ����������Ϊx��
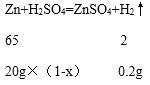
![]() x=67.5%��
x=67.5%��
�𣺣�1����ͭ��ĩ��ȫ��Ӧ����������������Ϊ0.2g��
��2���û�ͭ��ͭ����������Ϊ67.5%��

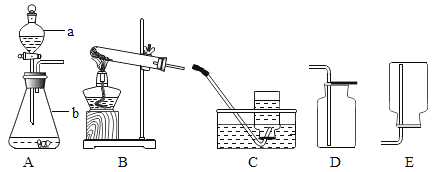
����Ŀ��Ϊ��֤�������ƵĻ�ѧ����ij��ȤС��ͬѧ������ͼ��ʾʵ�顣�����ʵ��ش�����
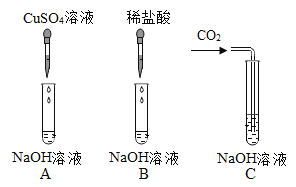
��1��A�Թ��й۲쵽��ʵ��������_____��
��2����ͬѧ�۲쵽B�Թ�������������Ȼ����B�Թ��м����μ�1��2����ɫ��̪��Һ������Һ��Ϊ��ɫ��������_____����������������������֤������������Һ��ϡ���ᷢ���˷�Ӧ��
��3��Ϊ����֤C�Թ�����̼�������ɣ���ͬѧ��Ƶ�ʵ�鷽�������ʾ�����������д������
���������ϣ�̼������Һ�Լ���
���� | ʵ�鲽�� | ʵ������ | ��Ӧ�Ļ�ѧ����ʽ |
һ | ��ȡ������Ӧ����Һ����һ֧�Թ��� �ڼ��������ϡ���� | _____ | _____ |
�� | ��_____ ��_____ | _____ | _____ |
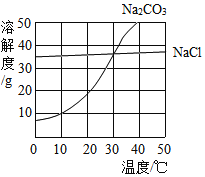
����Ŀ���±��� 20��ʱ�������ʵ��ܽ�����ݡ�
���� | Ca��OH��2 | NaOH | CaCO3 | Ca��HCO3��2 | Na2CO3 | NaHCO3 |
������/g | 0.16 | 109 | 0.0065 | 16.6 | 21.8 | 9.6 |
��1��������Һ������ 50g11%����������Һ�Ļ��������ǣ���������ȡ�������ƹ�������ȡˮ���ܽ���װƿ����ǩ�� ��������ƽ��ȡ�������ƹ��������_________ g��
��2����������̽��ʵ�飬20��ʱ���������ݻش���������:
����ϡ����������Һ��ͨ�� CO2�������� Na2CO3������ͨ�� CO2��Na2CO3 ��ת��Ϊ NaHCO3,��֪����Ϊ���Ϸ�Ӧ����д���û��Ϸ�Ӧ�Ļ�ѧ����ʽ��_________�� ���� 20��ʱ���� Na2CO3 ��Һ��ͨ������� CO2���ɹ۲쵽��������_________��
����ϱ����е��й����ݣ����� 20��ʱ��100g ������Һ���� CO2 ���������Ϊ���ݣ����ȥ CO �����е� CO2 ���ʣ�Ӧѡ��_________��Һ���ѧʽ����������CO2����Ĵ��ڣ���Ӧѡ��_________��Һ���ѧʽ����
�۽� 2.2g CO2 ͨ�� 47.8g һ��Ũ�ȵ� NaOH��Һ�г�ַ�Ӧ��Ӧ������û��CO2 �ݳ���ˮҲû������������Һ�е����ʽ��� Na2CO3�� NaHCO3 �������ʣ�NaHCO3 ��ˮ�е���� Na+�� HCO3-�������ʱ��Һ��̼Ԫ�ص���������Ϊ__________���ڴ˷�Ӧ�����У�����Ϊ�����ӵĸ���_________�����仯������д�� �л���û�У�
��3���絼�ʴ������ɸ���̽�����ֽⷴӦ��ʵ�ʡ���ͬ�����£�����Ũ��Խ�絼��Խ����Һ������Խǿ�������з�̪�� Ba(OH)2 ��Һƽ���ֳ��������������ձ��� ������絼�ʴ�������������һ�ݵμ�ϡ���ᣬ����һ�ݵμ���������Һ���μӹ����У���������Һ�ĵμ�����ʼ����ͬ�������Һ�ĵ絼�ʱ仯��ͼ��ʾ������˵����ȷ����__________
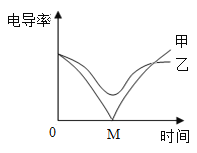
a�������ߵ絼�ʼ�С�����У���Һ�ɺ�ɫ��Ϊ��ɫ
b�������߶�Ӧ�ķ�Ӧ������������Ŀ������
c�������߶�Ӧ���������������Ʒ�Ӧ
d���������ϵ� M �����������Һǡ����ȫ��Ӧ